Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q2 (27 pts). Nitrate ion in potable water can be determined by measuring the absorbance of the water at 220nm in an ultraviolet-visible (UV-vis) spectrometer.
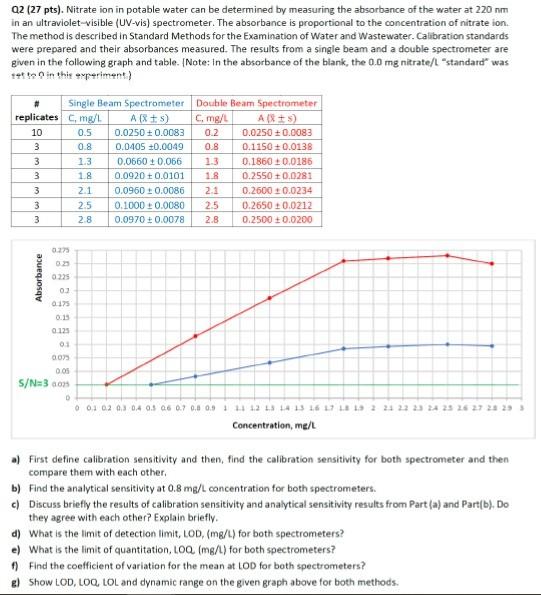
Q2 (27 pts). Nitrate ion in potable water can be determined by measuring the absorbance of the water at 220nm in an ultraviolet-visible (UV-vis) spectrometer. The absorbance is proportional to the concentration of nitrate ion. The method is described in Standard Methods for the Examination of Water and Wastewater. Calibration standards were prepared and their absorbances measured. The results from a single beam and a double spectrometer are given in the following graph and table. [Note: In the absorbance of the blank, the 0.0mg nitrate/l "standard" was tet ts 0 in thit experiment] a) First define calibration sensitivity and then, find the calibration sensitivity for both spectrometer and then compare them with each other. b) Find the analytical sensitivity at 0.8mg/L concentration for both spectrometers. c) Discuss briefly the results of calibration sensitivity and analytical sensitivity results from Part (a) and Part(b), Do they agree with each other? Explain briefly. d) What is the limit of detection limit, LOD, ( mg/L) for both spectrometers? e) What is the limit of quantitation, LOQ(mg/t) for both spectrometers? f) Find the coefficient of variation for the mean at LOD for both spectrometers? g) Show LOD, LOQ, LOL and dynamic range on the given graph above for both methods
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


