Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q2 (45%). The melting (fusion) points of pure Germanium and pure Silicon are 940C and 14100C, respectively, and are with fully miscible in both
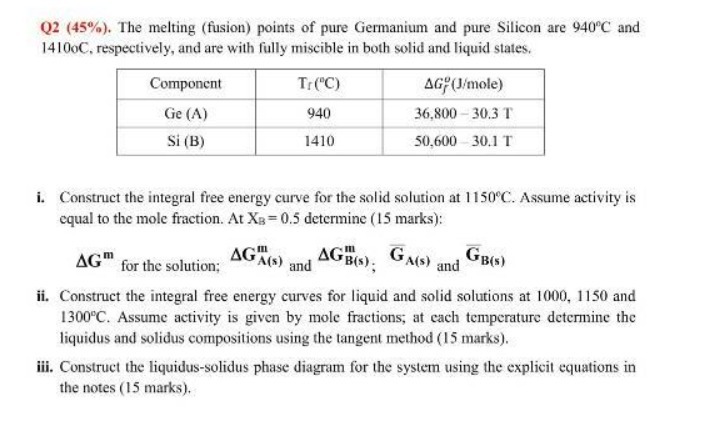
Q2 (45%). The melting (fusion) points of pure Germanium and pure Silicon are 940C and 14100C, respectively, and are with fully miscible in both solid and liquid states. Component Ge (A) Si (B) Tr (C) 940 1410 AG (J/mole) 36,800 30.3 T 50,600 30.1 T i. Construct the integral free energy curve for the solid solution at 1150C. Assume activity is equal to the mole fraction. At XB=0.5 determine (15 marks): AGA(s) and AGB(s); GA(S) GB(s) and AG for the solution; ii. Construct the integral free energy curves for liquid and solid solutions at 1000, 1150 and 1300C. Assume activity is given by mole fractions; at each temperature determine the liquidus and solidus compositions using the tangent method (15 marks). iii. Construct the liquidus-solidus phase diagram for the system using the explicit equations in the notes (15 marks).
Step by Step Solution
★★★★★
3.31 Rating (142 Votes )
There are 3 Steps involved in it
Step: 1
i To construct the integral free energy curve for the solid solution at 1150C we can use the equatio...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


