Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q2. (50 p) A high molecular weight hydrocarbon is decomposed into small molecules thermally in a 0.1 L BMR. The gas phase reaction stoichiometry can
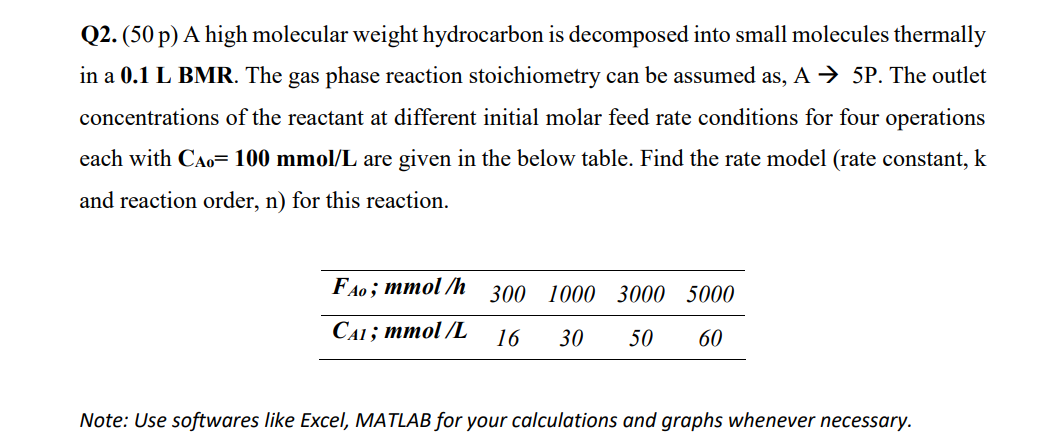 Q2. (50 p) A high molecular weight hydrocarbon is decomposed into small molecules thermally in a 0.1 L BMR. The gas phase reaction stoichiometry can be assumed as, A 5P. The outlet concentrations of the reactant at different initial molar feed rate conditions for four operations each with CA0=100mmol/L are given in the below table. Find the rate model (rate constant, k and reaction order, n ) for this reaction. Note: Use softwares like Excel, MATLAB for your calculations and graphs whenever necessary
Q2. (50 p) A high molecular weight hydrocarbon is decomposed into small molecules thermally in a 0.1 L BMR. The gas phase reaction stoichiometry can be assumed as, A 5P. The outlet concentrations of the reactant at different initial molar feed rate conditions for four operations each with CA0=100mmol/L are given in the below table. Find the rate model (rate constant, k and reaction order, n ) for this reaction. Note: Use softwares like Excel, MATLAB for your calculations and graphs whenever necessary Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


