Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q2. A liquid phase chemical reaction AR is carried out in a MFR under SS condition. A side product S is also formed in the
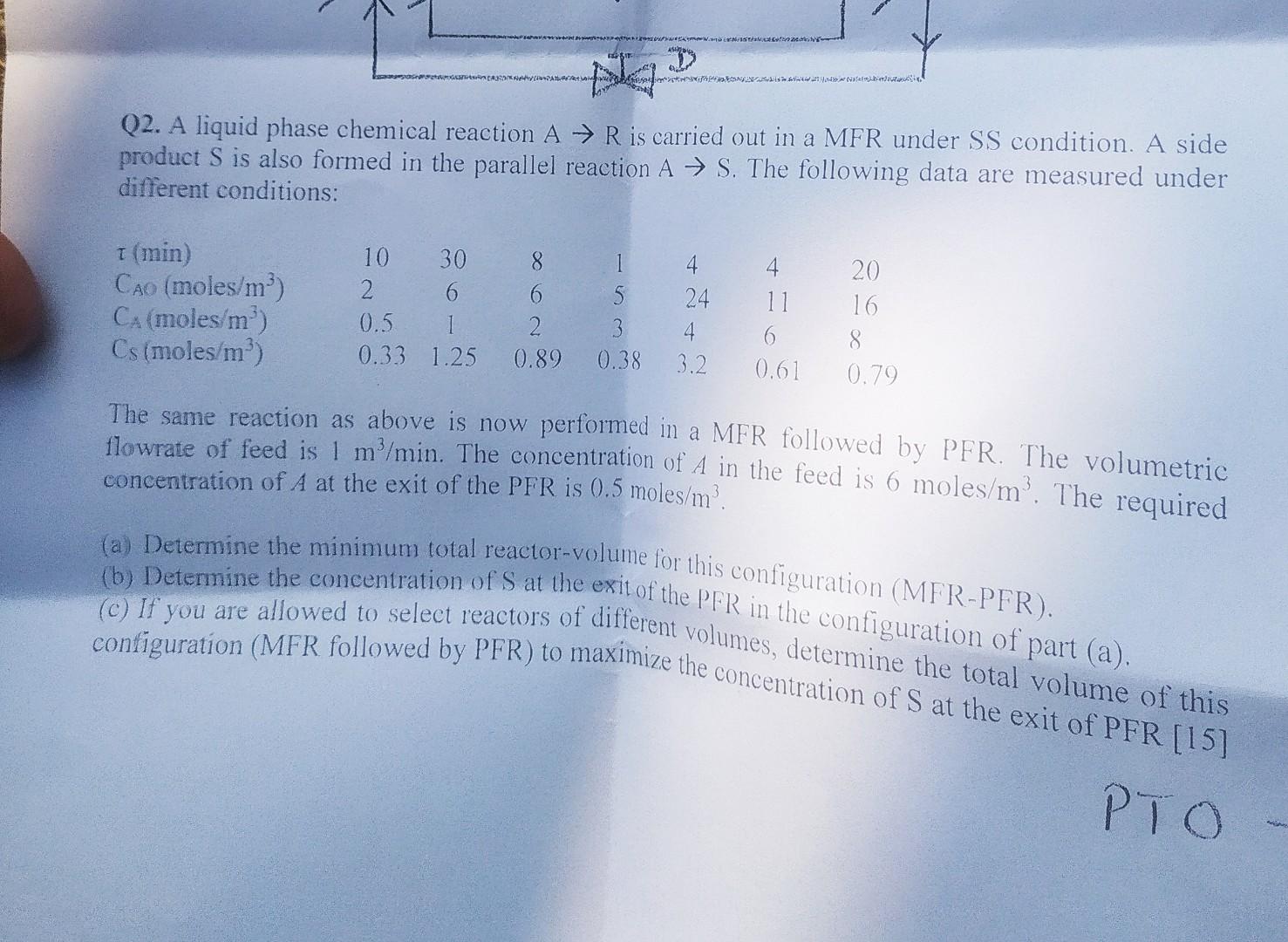
Q2. A liquid phase chemical reaction AR is carried out in a MFR under SS condition. A side product S is also formed in the parallel reaction AS. The following data are measured under different conditions: The same reaction as above is now performed in a MFR followed by PFR. The volumetric flowrate of feed is 1m3/min. The concentration of A in the feed is 6moles/m3. The required concentration of A at the exit of the PFR is 0.5moles/m3. (a) Determine the minimum total reactor-volume for this configuration (MFR-PFR). (b) Determine the concentration of S at the exit of the PFR in the configuration of part (a). (c) If you are allowed to select reactors of different volumes, determine the total volume of this - S at the exit of PFR[15]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


