Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q2. n-Heptane is dehydrocyclicized to toluene and hydrogen in a continuous vapour-phase reaction: C7H16C6H5CH3+4H2 Pure heptane at 400C is fed to the reactor. The reactor
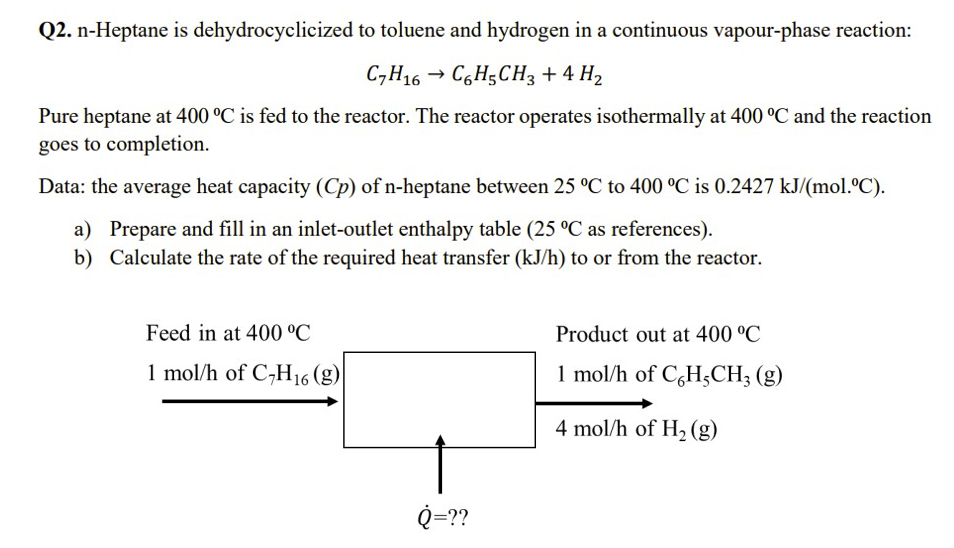 Q2. n-Heptane is dehydrocyclicized to toluene and hydrogen in a continuous vapour-phase reaction: C7H16C6H5CH3+4H2 Pure heptane at 400C is fed to the reactor. The reactor operates isothermally at 400C and the reaction goes to completion. Data: the average heat capacity (Cp) of n-heptane between 25C to 400C is 0.2427kJ/(mol.C). a) Prepare and fill in an inlet-outlet enthalpy table ( 25C as references). b) Calculate the rate of the required heat transfer (kJ/h) to or from the reactor
Q2. n-Heptane is dehydrocyclicized to toluene and hydrogen in a continuous vapour-phase reaction: C7H16C6H5CH3+4H2 Pure heptane at 400C is fed to the reactor. The reactor operates isothermally at 400C and the reaction goes to completion. Data: the average heat capacity (Cp) of n-heptane between 25C to 400C is 0.2427kJ/(mol.C). a) Prepare and fill in an inlet-outlet enthalpy table ( 25C as references). b) Calculate the rate of the required heat transfer (kJ/h) to or from the reactor Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


