Question
Q3: (20 points) The first three diffraction angles of the XRD pattem are observed using Cu K alphs (0.14506 nm) at 20 = 38.6,
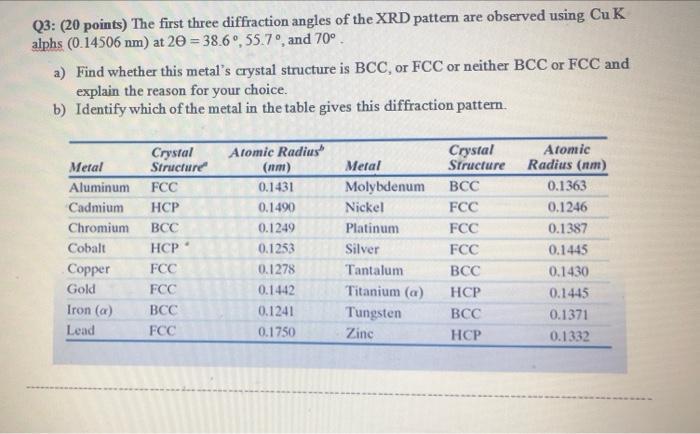
Q3: (20 points) The first three diffraction angles of the XRD pattem are observed using Cu K alphs (0.14506 nm) at 20 = 38.6, 55.7, and 70. a) Find whether this metal's crystal structure is BCC, or FCC or neither BCC or FCC and explain the reason for your choice. b) Identify which of the metal in the table gives this diffraction pattern. Crystal Structure Metal Aluminum FCC Cadmium HCP Chromium BCC HCP FCC FCC Cobalt Copper Gold Iron (a) Lead BCC FCC Atomic Radius (nm) 0.1431 0.1490 0.1249 0.1253 0.1278 0.1442 0.1241 0.1750 Metal Molybdenum Nickel Platinum Silver Tantalum Titanium (a) Tungsten Zinc Crystal Structure BCC FCC FCC FCC BCC HCP BCC HCP Atomic Radius (nm) 0.1363 0.1246 0.1387 0.1445 0.1430 0.1445 0.1371 0.1332
Step by Step Solution
3.33 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1
20 36 557 700 sin 0 109 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Materials Science and Engineering An Introduction
Authors: William D. Callister Jr., David G. Rethwisch
9th edition
978-1118546895, 111854689X, 978-1118477700, 1118477707, 1118324579, 978-1118324578
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



