Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q3. A 1 mol ideal gas in a cylinder with a frictionless piston is initially at 25C and 1 bar. The ideal gas has a
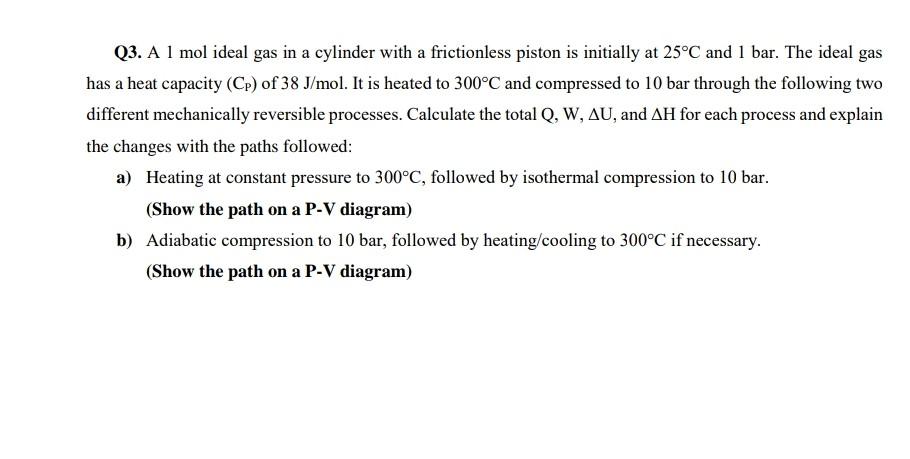
Q3. A 1 mol ideal gas in a cylinder with a frictionless piston is initially at 25C and 1 bar. The ideal gas has a heat capacity (CP) of 38J/mol. It is heated to 300C and compressed to 10 bar through the following two different mechanically reversible processes. Calculate the total Q,W,U, and H for each process and explain the changes with the paths followed: a) Heating at constant pressure to 300C, followed by isothermal compression to 10 bar. (Show the path on a P-V diagram) b) Adiabatic compression to 10bar, followed by heating/cooling to 300C if necessary. (Show the path on a P-V diagram)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


