Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q3. A gas mixture containing 40% mole fraction of Methane (CH4,MW=16.04kg/kmol) and the palance Butane (C4H10,MW=58.1kg/kmol) enters a combustion chamber at a temperature of 60C
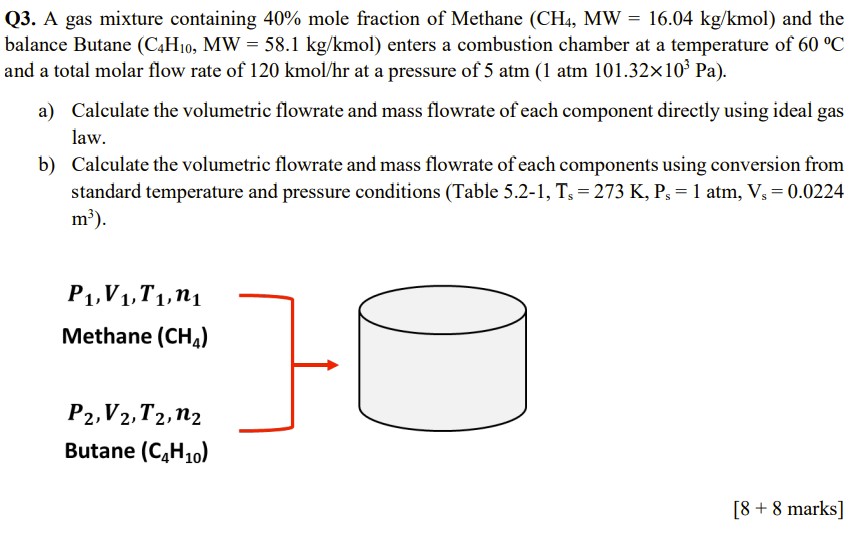 Q3. A gas mixture containing 40% mole fraction of Methane (CH4,MW=16.04kg/kmol) and the palance Butane (C4H10,MW=58.1kg/kmol) enters a combustion chamber at a temperature of 60C and a total molar flow rate of 120kmol/hr at a pressure of 5atm(1atm101.32103Pa). a) Calculate the volumetric flowrate and mass flowrate of each component directly using ideal gas law. b) Calculate the volumetric flowrate and mass flowrate of each components using conversion from standard temperature and pressure conditions (Table 5.2-1, Ts=273K,Ps=1atm,Vs=0.0224 m3 )
Q3. A gas mixture containing 40% mole fraction of Methane (CH4,MW=16.04kg/kmol) and the palance Butane (C4H10,MW=58.1kg/kmol) enters a combustion chamber at a temperature of 60C and a total molar flow rate of 120kmol/hr at a pressure of 5atm(1atm101.32103Pa). a) Calculate the volumetric flowrate and mass flowrate of each component directly using ideal gas law. b) Calculate the volumetric flowrate and mass flowrate of each components using conversion from standard temperature and pressure conditions (Table 5.2-1, Ts=273K,Ps=1atm,Vs=0.0224 m3 ) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


