Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q3 The Ottsel engine has been proposed by an inventor who built a very complicated prototype of its engine. However, his knowledge of thermodynamics
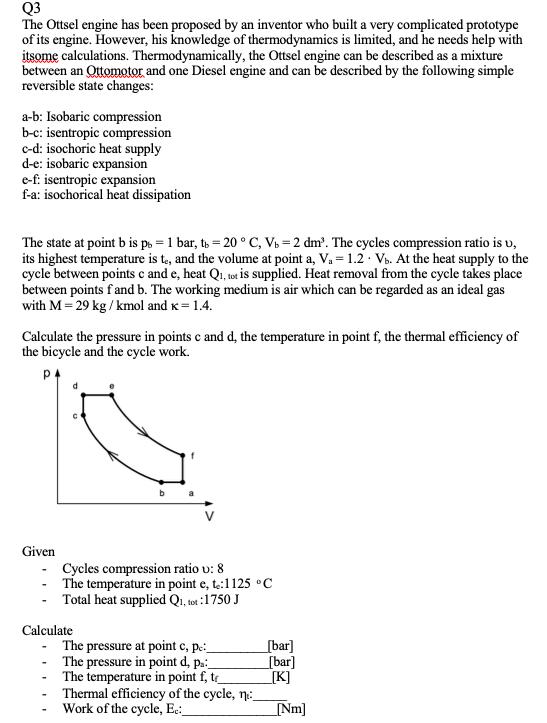
Q3 The Ottsel engine has been proposed by an inventor who built a very complicated prototype of its engine. However, his knowledge of thermodynamics is limited, and he needs help with itsome calculations. Thermodynamically, the Ottsel engine can be described as a mixture between an Ottomotor and one Diesel engine and can be described by the following simple reversible state changes: a-b: Isobaric compression b-c: isentropic compression c-d: isochoric heat supply d-e: isobaric expansion e-f. isentropic expansion f-a: isochorical heat dissipation The state at point b is ps = 1 bar, ts = 20 C, V. = 2 dm'. The cycles compression ratio is v, its highest temperature is te, and the volume at point a, V. = 1.2 Vo. At the heat supply to the cycle between points c and e, heat Qi, tot is supplied. Heat removal from the cycle takes place between points fand b. The working medium is air which can be regarded as an ideal gas with M = 29 kg / kmol and x= 1.4. Calculate the pressure in points c and d, the temperature in point f, the thermal efficiency of the bicycle and the cycle work. Given Cycles compression ratio u: 8 The temperature in point e, t.:1125 C Total heat supplied Q., tot :1750 J Calculate The pressure at point c, pe: The pressure in point d, p.: The temperature in point f, te Thermal efficiency of the cycle, n: Work of the cycle, Ec_ [bar] [bar] [K] Nm]
Step by Step Solution
★★★★★
3.51 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


