Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q4 (a) According to the Figure Q4(a), n-Butane fuel (CH) is burned with a 100 % excess air. (i) Identify the mole fractions of each
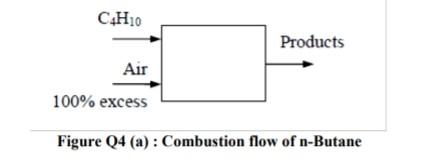
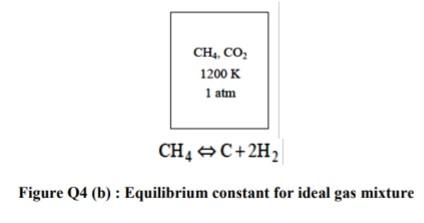
Q4 (a) According to the Figure Q4(a), n-Butane fuel (CH) is burned with a 100 % excess air. (i) Identify the mole fractions of each product (8 marks) (ii) Calculate the mass of carbon dioxide in the products per unit mass of the fuel (2 marks) (iii) Evaluate the air-fuel ratio (2 marks) (b) According to the Figure Q4(b), a gaseous mixture of 30 percent (by mole fraction) methane and 70 percent carbon dioxide is heated at 1 atm pressure up to 1200 K. The natural logarithm of the equilibrium constant for the reaction at 1200 K is 4.147. Analyze the equilibrium composition (by mole fraction) of the resulting mixtures. (13 marks) CH10 Products Air 100% excess Figure Q4 (a) : Combustion flow of n-Butane CH, CO 1200 K 1 atm CH4 C+2H2 Figure Q4 (b): Equilibrium constant for ideal gas mixture
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


