Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Use the equilibrium data of Acetone (Ac) and Chloroform (Chl) at 35C given in the Figure Q4 below: i. Find the vapor pressures of
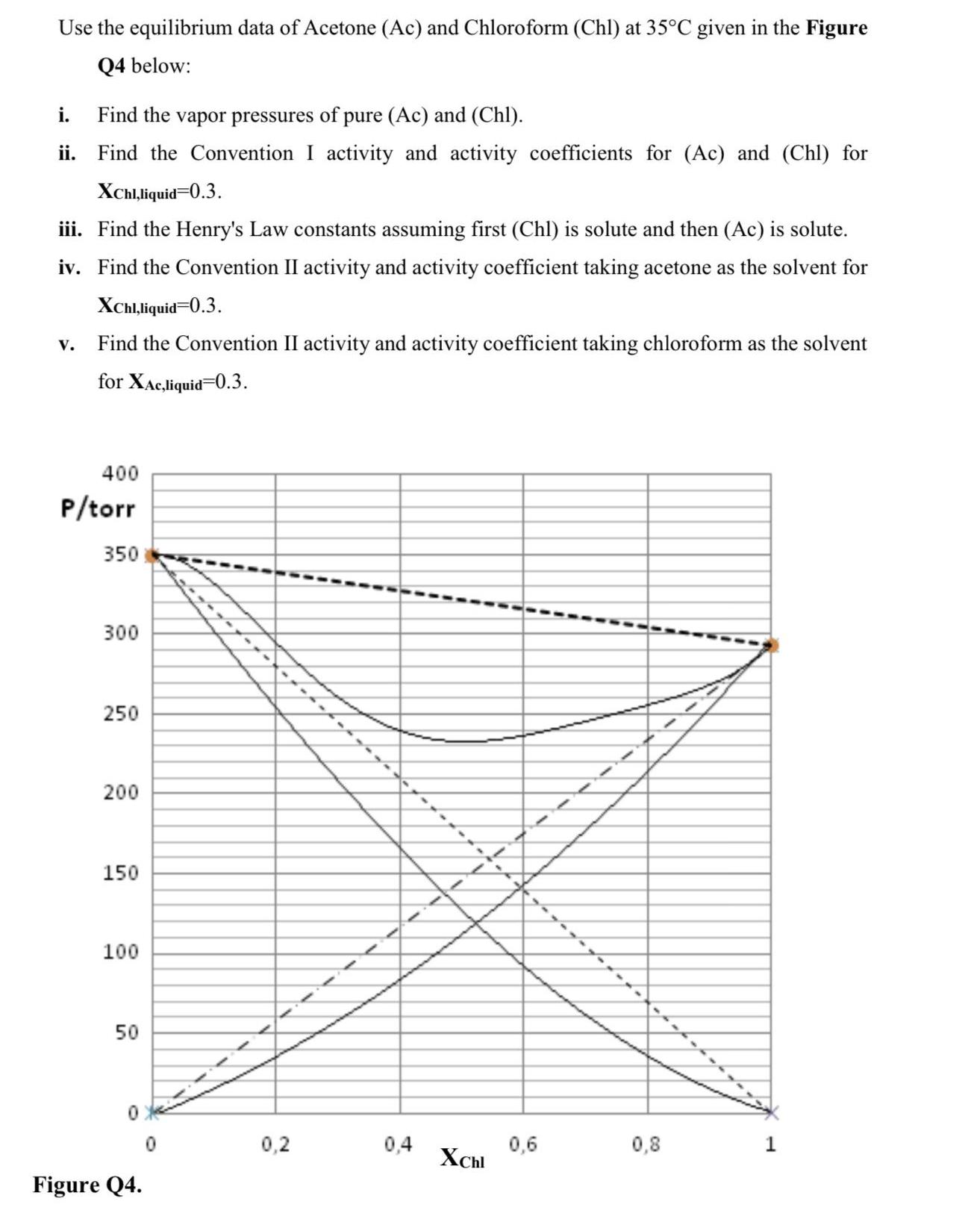
Use the equilibrium data of Acetone (Ac) and Chloroform (Chl) at 35C given in the Figure Q4 below: i. Find the vapor pressures of pure (Ac) and (Chl). ii. Find the Convention I activity and activity coefficients for (Ac) and (Chl) for XChl,liquid=0.3. iii. Find the Henry's Law constants assuming first (Chl) is solute and then (Ac) is solute. iv. Find the Convention II activity and activity coefficient taking acetone as the solvent for XChl,liquid=0.3. V. Find the Convention II activity and activity coefficient taking chloroform as the solvent for XAc,liquid=0.3. 400 P/torr 350 300 250 200 150 100 50 Figure Q4. 0,2 0,4 XChl 0,6 0,8
Step by Step Solution
★★★★★
3.38 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
To find the various properties and coefficients as requested we can use the given equilibrium data for Acetone Ac and Chloroform Chl at 35C It appears ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


