Answered step by step
Verified Expert Solution
Question
1 Approved Answer
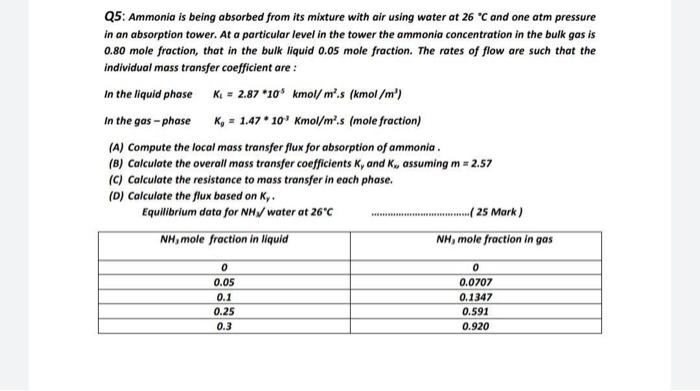
Q5: Ammonia is being absorbed from its mixture with air using water at 26 C and one atm pressure in an absorption tower. At a
Q5: Ammonia is being absorbed from its mixture with air using water at 26 C and one atm pressure in an absorption tower. At a particular level in the tower the ammonia concentration in the bulk gas is 0.80 mole fraction, that in the bulk liquid 0.05 mole fraction. The rates of flow are such that the individual mass transfer coefficient are : In the liquid phase K = 2.87 *105 kmol/ m.s (kmol/m) In the gas-phase Kg = 1.47 * 10 Kmol/m.s (mole fraction) (A) Compute the local mass transfer flux for absorption of ammonia. (B) Calculate the overall mass transfer coefficients K, and Kx, assuming m = 2.57 (C) Calculate the resistance to mass transfer in each phase. (D) Calculate the flux based on Ky. Equilibrium data for NH3/ water at 26C NH3 mole fraction in liquid 0 0.05 0.1 0.25 0.3 .( 25 Mark) NH, mole fraction in gas 0 0.0707 0.1347 0.591 0.920
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


