Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q5- Consider the following disaccharide: CH2OH 0 OH H H OH H O H H OH CH2 0 H H H OH H OH OH
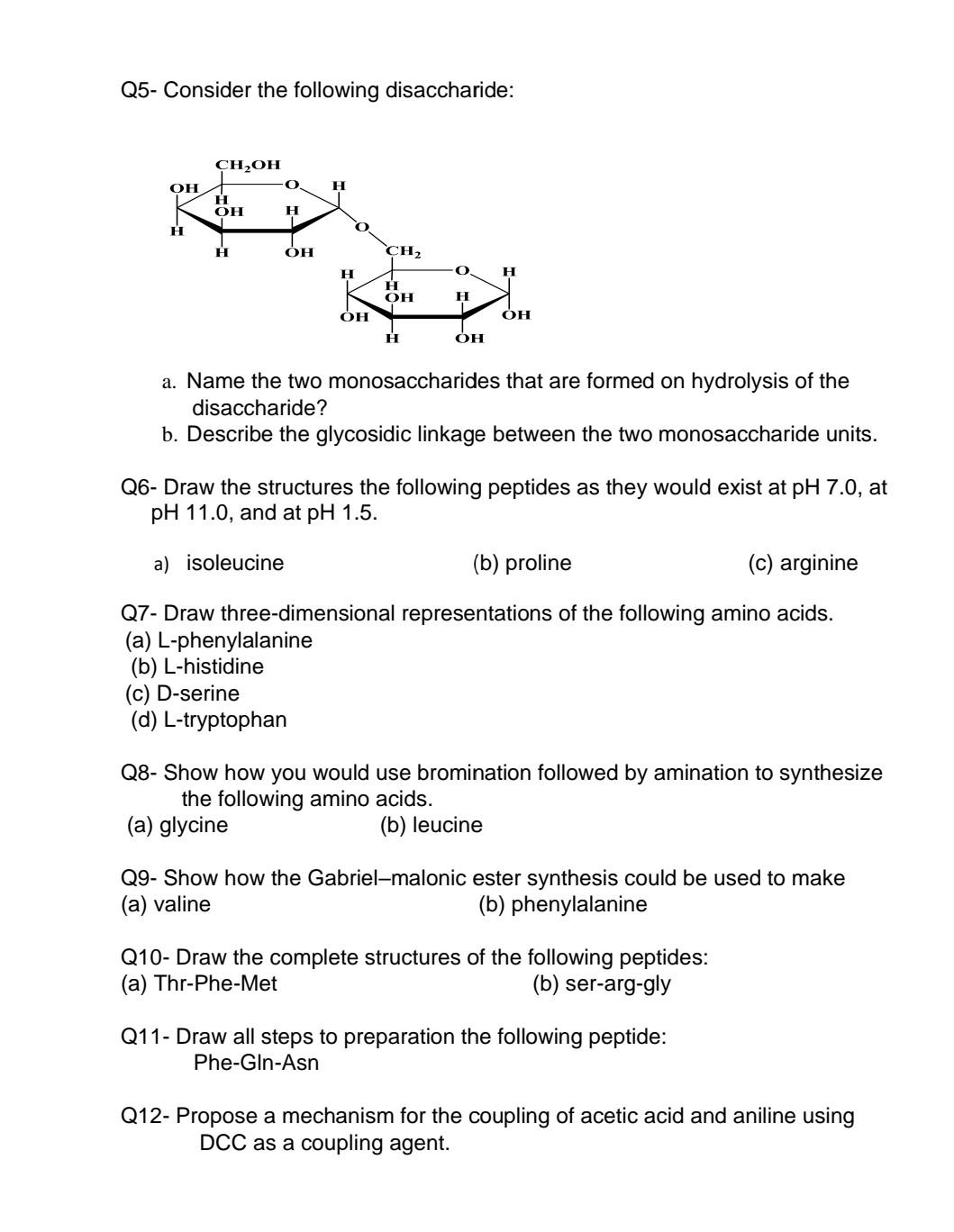
Q5- Consider the following disaccharide: CH2OH 0 OH H H OH H O H H OH CH2 0 H H H OH H OH OH H OH a. Name the two monosaccharides that are formed on hydrolysis of the disaccharide? b. Describe the glycosidic linkage between the two monosaccharide units. Q6- Draw the structures the following peptides as they would exist at pH 7.0, at pH 11.0, and at pH 1.5. a) isoleucine (b) proline (c) arginine Q7- Draw three-dimensional representations of the following amino acids. (a) L-phenylalanine (b) L-histidine (C) D-serine (d) L-tryptophan Q8- Show how you would use bromination followed by amination to synthesize the following amino acids. (a) glycine (b) leucine Q9- Show how the Gabriel-malonic ester synthesis could be used to make (a) valine (b) phenylalanine Q10- Draw the complete structures of the following peptides: (a) Thr-Phe-Met (b) ser-arg-gly Q11- Draw all steps to preparation the following peptide: Phe-Gln-Asn Q12- Propose a mechanism for the coupling of acetic acid and aniline using DCC as a coupling agent
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


