Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Qa/ By using Fig (1-1)3 Fig(2-1) and the data in table below. Temp. t %vol. Distilate 50 IBP: 0 220 20 290 40 60 80

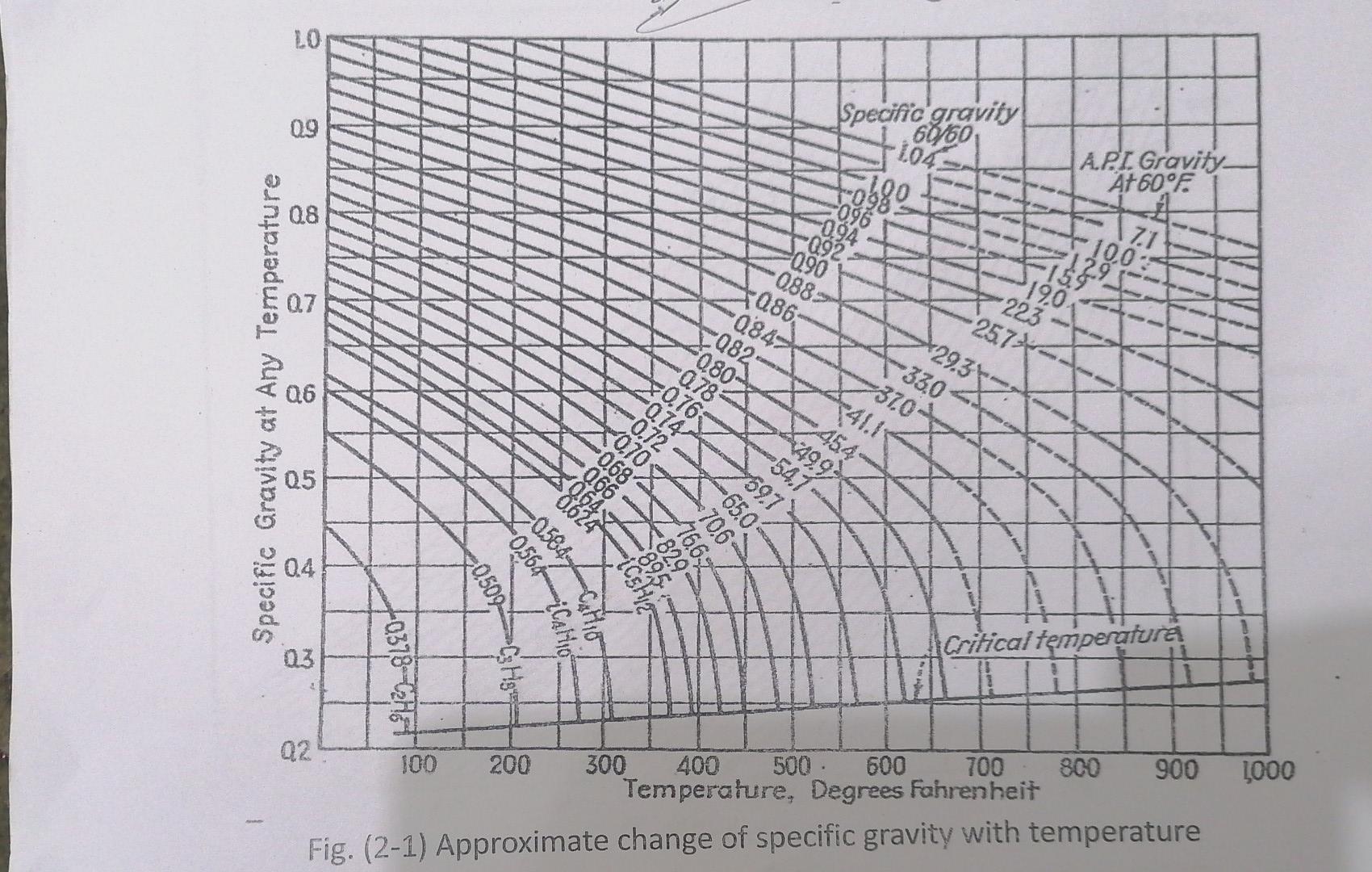
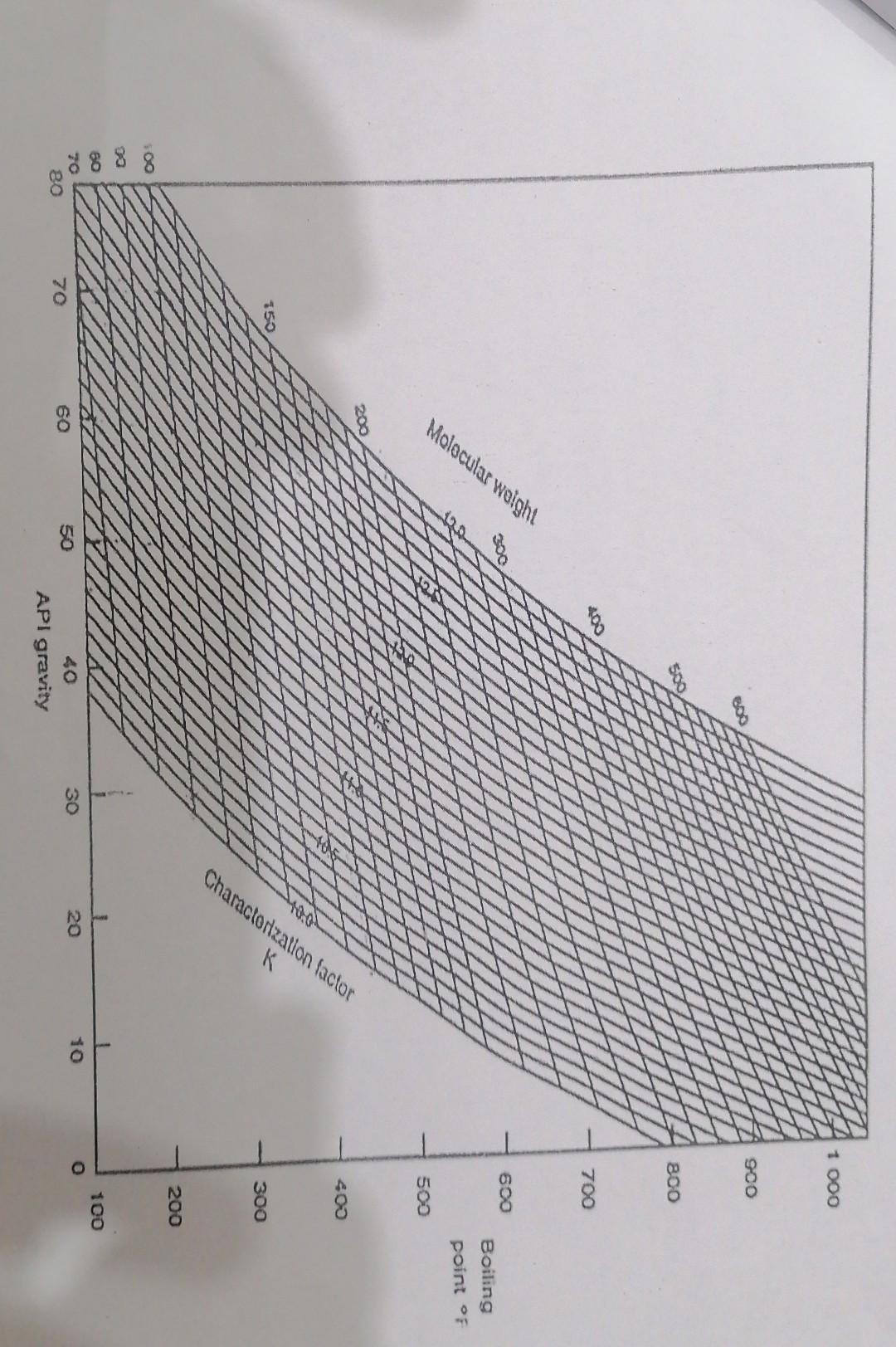
Qa/ By using Fig (1-1)3 Fig(2-1) and the data in table below. Temp. t %vol. Distilate 50 IBP: 0 220 20 290 40 60 80 90 350 420 500 540 EBP: 95 |- Estimate the specifiegowity 6% for fraction has Apl=37 by using Fig 2-1. 2 - Estimate the specific growity at 500 F for the Same fruetion APS-37 3. Estimate the kw value from Fig 1-1, ond origin of crude oil. 4- Estimate the Molecular weight from Fig 1-). 5- Calculate the kw by wattson equation. 6- Calculate the corelation Index (C.I), and ovigin of crude oil. LO 09 Specific gravity 60060. 104. API. Gravity At 60F 096 08 10680 289038 088 0.7 F986- 084 190 223 - 257* 082 080 29.3 -330 370- 141.1 Q6 Specific Gravity at Any Temperature *49.92 454 -541 597 Q5 Dog ond 49 -65.0 0584 GOL V Eggh 04 -0564 0500 MU iCalio Critical temperatura 03 40378 Chat CHg4 1000 Q2 100 200 300 400 500 600 700 800 900 Temperature, Degrees Fahrenheit Fig. (2-1) Approximate change of specific gravity with temperature 1 000 SOO 500 BOO 400 700 Molecular weight 600 Boiling point of 500 - 400 Characterization factor 150 300 200 7888 1 10 100 20 O 30 50 70 80 60 API gravity
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


