Answered step by step
Verified Expert Solution
Question
1 Approved Answer
quantum engineering (a) Consider an electron in a solid, which could be a semiconductor or an insulator. We will exclude a metal since the potential

quantum engineering
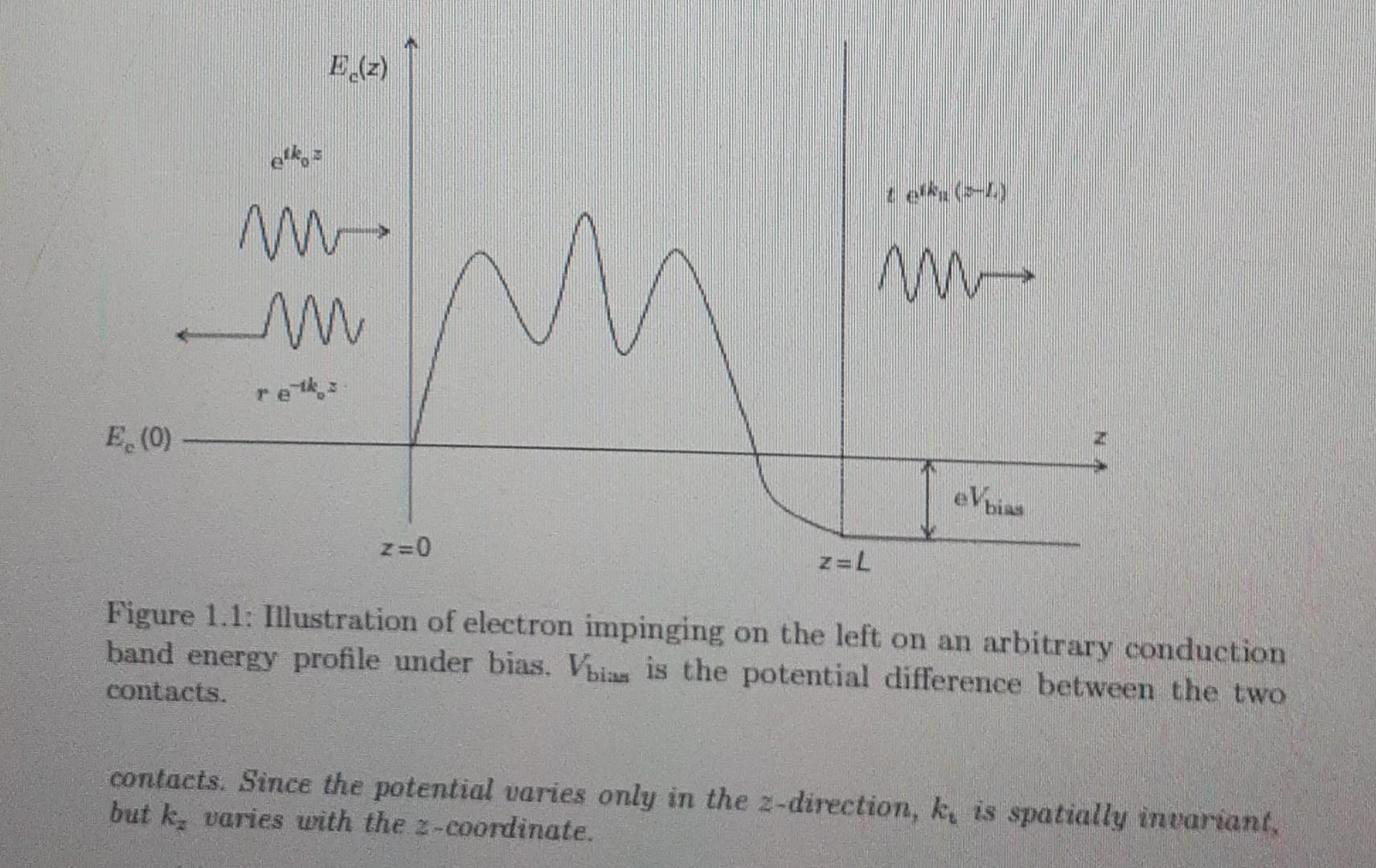
(a) Consider an electron in a solid, which could be a semiconductor or an insulator. We will exclude a metal since the potential inside a metal is usually spatially invariant as a metal cannot sustain an electric field. The potential that an electron sees inside a semiconductor or insulator is the conduction band profile. We will assume that it is time independent and varies only along one direction, which we call the 2-direction (see Figure 1.1). The effective mass also varies along that direction. Show that the stationary solutions of the Schrdinger equation obey the equation t? d h2 2m*(z) | dr? var, y, z) 1 d m*(z) dz V(2. y. 2) dy? + Ec(z)v(r, y, z) = Ev(r, y, z), (1.1) where Ec(z) is the conduction band edge and E is the total energy of the electron, which is independent of 2 because the total energy is a good quantum number in the absence of dissipation. In Equation (1.1). E = Ekin(-) + E-(-) and Ekin (z) = (K?(z) +13) 2m: (1.2) where k, and kt k+ k are the longitudinal and transverse components of the electron's wave vector, respectively, while m = m*(0) is the effective mass in the E.(z) elke M M - E.(0) eVbiss Z=0 Figure 1.1: Illustration of electron impinging on the left on an arbitrary conduction band energy profile under bias. Vbian is the potential difference between the two contacts. contacts. Since the potential varies only in the 2-direction, ky is spatially invariant, but k, varies with the 2-coordinateStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


