Answered step by step
Verified Expert Solution
Question
1 Approved Answer
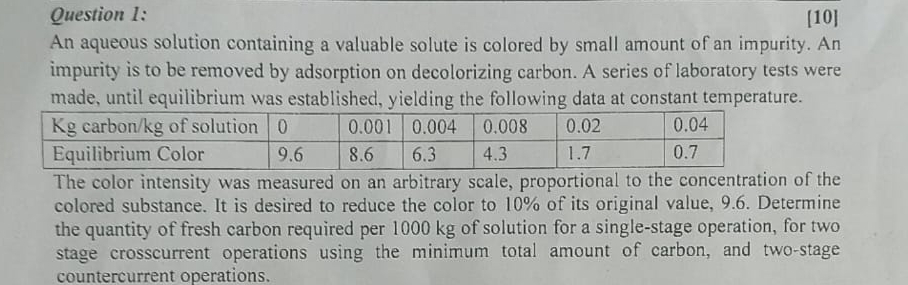
Question 1 : [ 1 0 ] An aqueous solution containing a valuable solute is colored by small amount of an impurity. An impurity is
Question :
An aqueous solution containing a valuable solute is colored by small amount of an impurity. An impurity is to be removed by adsorption on decolorizing carbon. A series of laboratory tests were made, until equilibrium was established, yielding the following data at constant temperature.
table carbon of solution,Equilibrium Color,
The color intensity was measured on an arbitrary scale, proportional to the concentration of the colored substance. It is desired to reduce the color to of its original value, Determine the quantity of fresh carbon required per of solution for a singlestage operation, for two stage crosscurrent operations using the minimum total amount of carbon, and twostage countercurrent operations.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


