Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 1 10 pts For a process with 1500 kJ of heat transfered out of a system at an interface temperature of 300K, what
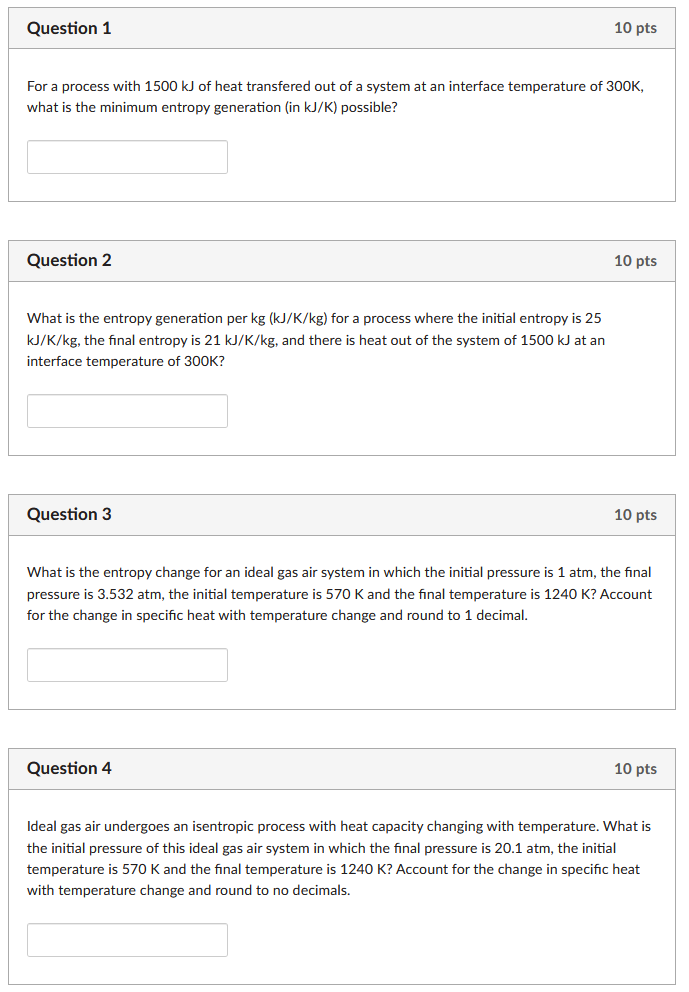
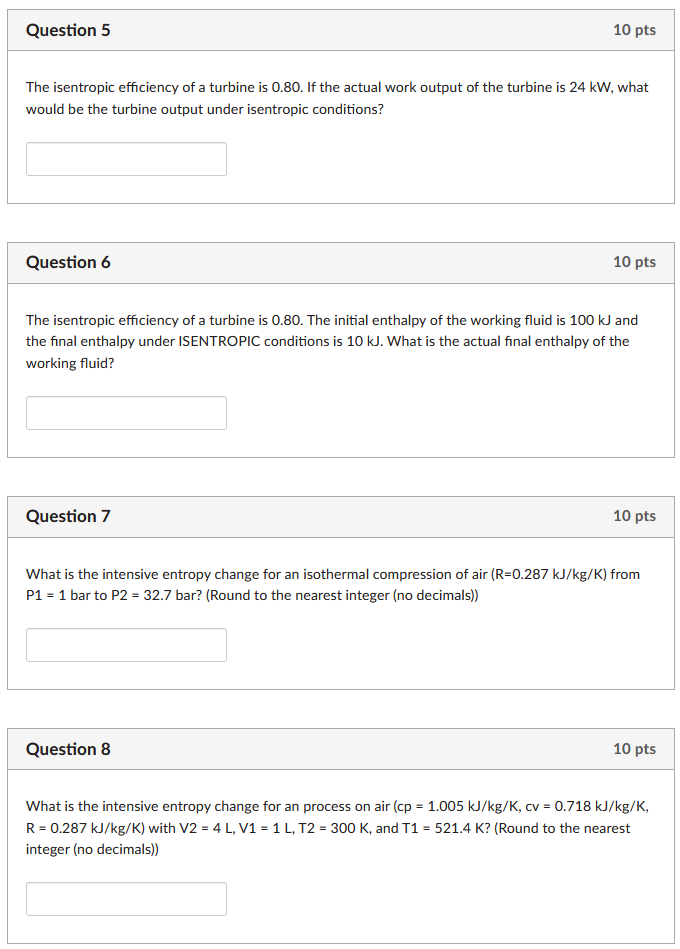
Question 1 10 pts For a process with 1500 kJ of heat transfered out of a system at an interface temperature of 300K, what is the minimum entropy generation (in kJ/K) possible? Question 2 What is the entropy generation per kg (kJ/K/kg) for a process where the initial entropy is 25 KJ/K/kg, the final entropy is 21 kJ/K/kg, and there is heat out of the system of 1500 kJ at an interface temperature of 300K? Question 3 10 pts 10 pts What is the entropy change for an ideal gas air system in which the initial pressure is 1 atm, the final pressure is 3.532 atm, the initial temperature is 570 K and the final temperature is 1240 K? Account for the change in specific heat with temperature change and round to 1 decimal. Question 4 10 pts Ideal gas air undergoes an isentropic process with heat capacity changing with temperature. What is the initial pressure of this ideal gas air system in which the final pressure is 20.1 atm, the initial temperature is 570 K and the final temperature is 1240 K? Account for the change in specific heat with temperature change and round to no decimals. Question 5 10 pts The isentropic efficiency of a turbine is 0.80. If the actual work output of the turbine is 24 kW, what would be the turbine output under isentropic conditions? Question 6 10 pts The isentropic efficiency of a turbine is 0.80. The initial enthalpy of the working fluid is 100 kJ and the final enthalpy under ISENTROPIC conditions is 10 kJ. What is the actual final enthalpy of the working fluid? Question 7 10 pts What is the intensive entropy change for an isothermal compression of air (R=0.287 kJ/kg/K) from P1 = 1 bar to P2 = 32.7 bar? (Round to the nearest integer (no decimals)) Question 8 10 pts What is the intensive entropy change for an process on air (cp = 1.005 kJ/kg/K, cv = 0.718 kJ/kg/K, R = 0.287 kJ/kg/K) with V2 = 4 L, V1 = 1 L, T2 = 300 K, and T1 = 521.4 K? (Round to the nearest integer (no decimals))
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


