Answered step by step
Verified Expert Solution
Question
1 Approved Answer
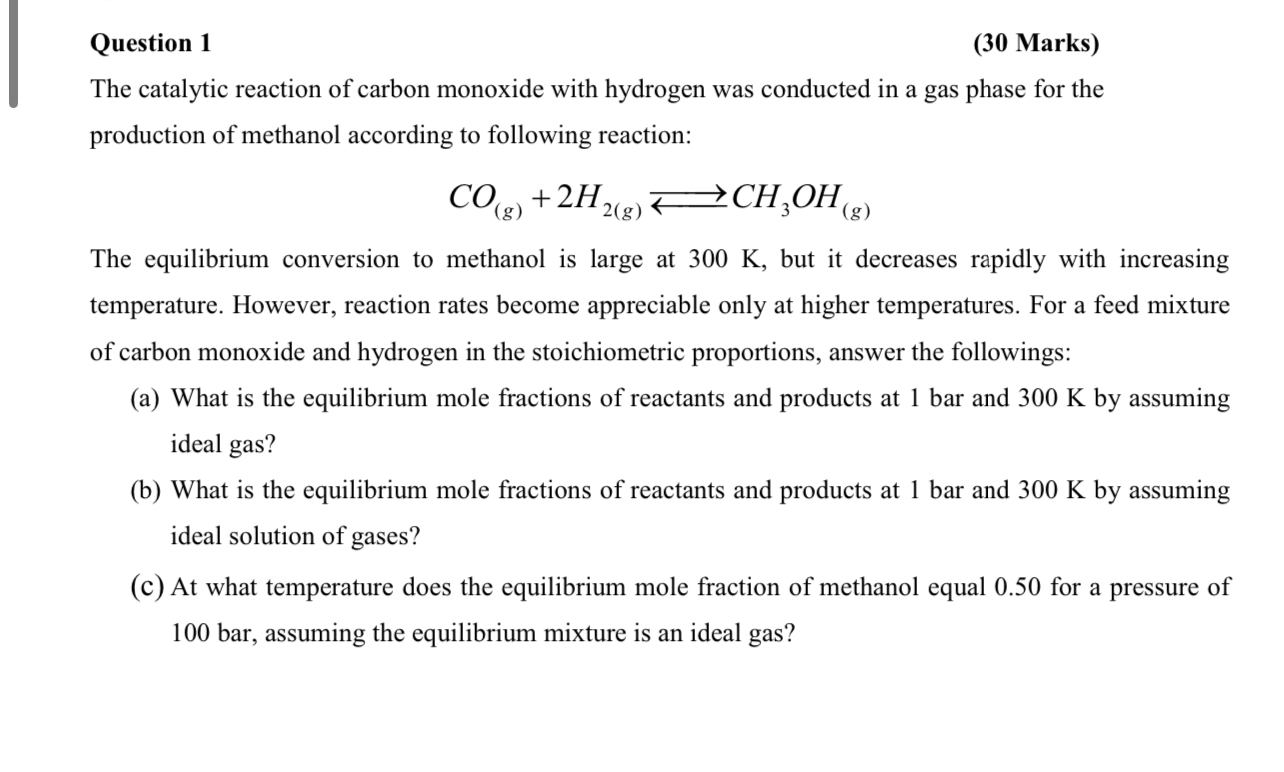
Question 1 ( 3 0 Marks ) The catalytic reaction of carbon monoxide with hydrogen was conducted in a gas phase for the production of
Question
Marks
The catalytic reaction of carbon monoxide with hydrogen was conducted in a gas phase for the production of methanol according to following reaction:
The equilibrium conversion to methanol is large at but it decreases rapidly with increasing temperature. However, reaction rates become appreciable only at higher temperatures. For a feed mixture of carbon monoxide and hydrogen in the stoichiometric proportions, answer the followings:
a What is the equilibrium mole fractions of reactants and products at bar and by assuming ideal gas?
b What is the equilibrium mole fractions of reactants and products at bar and by assuming ideal solution of gases?
c At what temperature does the equilibrium mole fraction of methanol equal for a pressure of assuming the equilibrium mixture is an ideal ga
Solve on paper please with clearly calculation
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


