Answered step by step
Verified Expert Solution
Question
1 Approved Answer
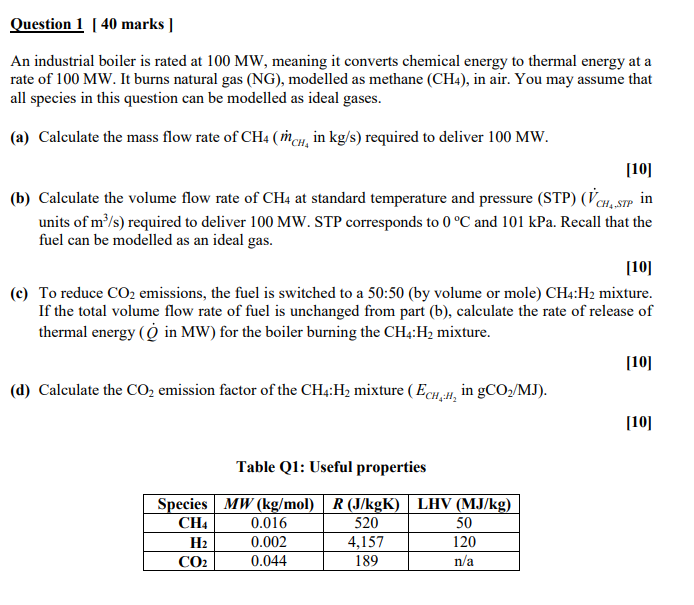
Question 1 [ 4 0 marks ] An industrial boiler is rated at 1 0 0 M W , meaning it converts chemical energy to
Question marks
An industrial boiler is rated at meaning it converts chemical energy to thermal energy at a
rate of It burns natural gas NG modelled as methane in air. You may assume that
all species in this question can be modelled as ideal gases.
a Calculate the mass flow rate of in : required to deliver
b Calculate the volume flow rate of at standard temperature and pressure STP in
units of required to deliver STP corresponds to and kPa. Recall that the
fuel can be modelled as an ideal gas.
c To reduce emissions, the fuel is switched to a :by volume or mole: mixture.
If the total volume flow rate of fuel is unchanged from part b calculate the rate of release of
thermal energy in MW for the boiler burning the : mixture.
d Calculate the emission factor of the : mixture in
Table Q: Useful properties
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


