Answered step by step
Verified Expert Solution
Question
1 Approved Answer
question 1, 4, and 5 I have the procedure for question 1 in these pictures The unbalanced chemical equation for this reaction is shown below.
question 1, 4, and 5 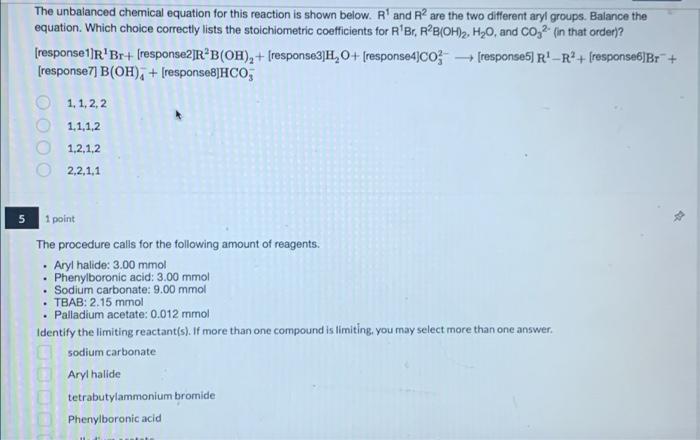



I have the procedure for question 1 in these pictures 

The unbalanced chemical equation for this reaction is shown below. R' and R are the two different aryl groups. Balance the equation. Which choice correctly lists the stoichiometric coefficients for R'Br. RB(OH)2, H20, and CO 2 in that order)? [response1]R'Br+ (response2]RB(OH)2 + (response3]H, 0+ (response4)co [response) R'-RP+ response6]Br + [response7) B(OH); + (response8]HCO, 1,1,2,2 1.1.1.2 1,2,1,2 2,2,1,1 . . 1 point The procedure calls for the following amount of reagents. Aryl halide: 3.00 mmol Phenylboronic acid: 3.00 mmol Sodium carbonate: 9.00 mmol TBAB: 2.15 mmol Palladium acetate: 0.012 mmol Identify the limiting reactant(s). If more than one compound is limiting, you may select more than one answer sodium carbonate Aryl halide tetrabutylammonium bromide Phenylboronic acid D Question 1 15 pts Balance the equation for the first reaction - the synthesis of 3- mtrophthalhydrazide from 3-nitrophthalic acid by filling in the stoichiometric coefficient. C3H5NO. (3-nitrophthalic acid) + H2NNH2 C3H5N304 (3- nitrophthalhydrazide) + H20. What is the limiting reactant? What is the theoretical yield of 3-nitrophthalhydrazide? moles Assuming 3-nitrophthalhydrazide is the limiting reactant for the second reaction, determine the theoretical yield of luminol: moles, which is grams. Chemicals: 3-nitrophthalic acid 8% aqueous hydrazine Triethylene glycol Water 3 M sodium hydroxide Sodium hydrosulfite Glacial acetic acid 3% aqueous potassium ferricyanide 3% aqueous hydrogen peroxide bleach Safety: Caution! Hydrazine is dangerous in many ways. We are using dilute aqueous solutions to minimize the hazard, but you should still treat hydrazine solutions as among the more hazardous things you have handled. Pure hydrazine is highly toxic and very unstable. In concentrated form it may cause any number of serious acute health problems. Pure hydrazine may explode if heated. Hydrazine is also corrosive. Take all of the precautions you would for concentrated sulfuric acid, as well as all of the precautions you would for a volatile liquid that you don't want to breathe Despite containing cyanide groups, potassium ferricyanide has minimal toxicity under neutral and basic conditions. Under strong acid conditions, hydrogen cyanide gas it evolved. Under no circumstances should you be handling strong acid today. All waste generated from this experiment must be thoroughly oxidized prior to disposal. Do not pour any waster or leftover chemicals into a waste bottle or down the drain. A waste treatment station will be set up in one of the hoods. Advice: This synthesis is pretty hard to mess up. Be careful handling hydrazine. The chemiluminescent reaction, however, is finickier Procedure: Work in groups of 2 or 3. Preparation of 3-nitrophthalhydrazide Heat a mixture of 1 g of 3-nitrophthalic acid and 2 mL of 8% aqueous hydrazine (Caution!) in a 50 mL round bottom flask until all of the solid is dissolved. Add 3 mL of triethylene glycol. Set up a simple distillation. Push the thermometer all the way down into the reaction mixture, but be careful not to let it touch the bottom of the flask, Distil until the solution reaches 200 C. Cool the mixture to about 100C. Meanwhile, heat 15 mL of water on a hot plate (don't let it boil away). When the reaction mixture is at or below 100 C (crystals may appear), add the hot water. Cool the flask on ice and collect the solid by vacuum filtration. Wash with water two or three times. Preparation of 3-aminophthalhydrazide Return the sold to the uncleaned flask in which it was prepared. Add 5 mL of 3 M NaOH and 3 g of sodium dithionite (may also be labeled sodium hydrosulfite) dihydrate. Wash the solid down the walls with a little bit of water. Boli the mixture for at least five minutes (the product may separate from solution). Add 2 ml glaciat acetic acid and cool on ice. Collect the precipitate. We will not purify or characterize luminal any further. Chemiluminescent reaction Prepare two solutions Solution A: dissolve your crude luminot in 2 mL of 3 M NaOH and dilute that mixture to a total volume of 20 mL Solution B: mix 4 mL of 3% aqueous potassium ferricyanide and 4 mL of 3% aqueous hydrogen peroxide and dilute the solution to a total volume of 40 ml. In a dark place, slowly and simultaneously pour solutions A and B together into a flask. Your instructor may have you dilute solutions A and B prior to mixing. Waste disposal Place all waste, including unused chemicals, in the large beaker set up in one of the hoods. The waste needs to be treated with bleach before disposal. No waste should go into the waste bottles or down the drain. Assignment: This experiment is a mandatory postlab 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


