Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 1 : a . Explain the effect of temperature on: ( Please Use Molecular Level Explanations! ) i . Vapor Pressure ii . Viscosity
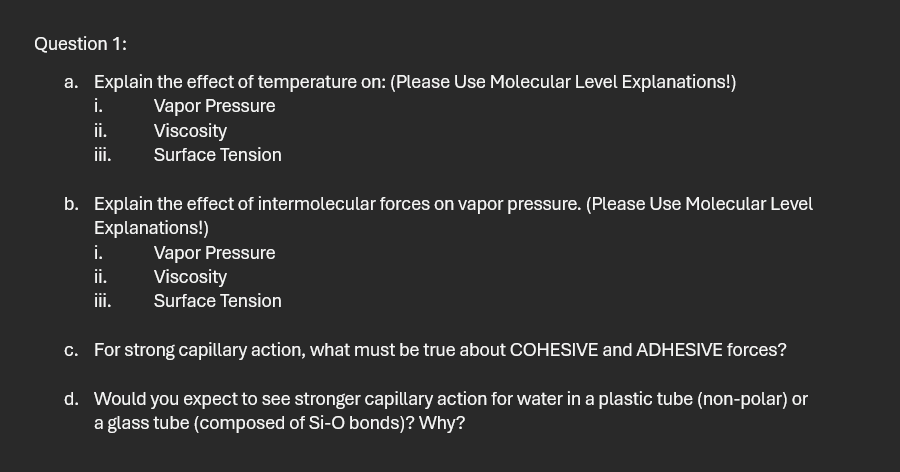
Question :
a Explain the effect of temperature on: Please Use Molecular Level Explanations!
i Vapor Pressure
ii Viscosity
iii. Surface Tension
b Explain the effect of intermolecular forces on vapor pressure. Please Use Molecular Level Explanations!
i Vapor Pressure
ii Viscosity
iii. Surface Tension
c For strong capillary action, what must be true about COHESIVE and ADHESIVE forces?
d Would you expect to see stronger capillary action for water in a plastic tube nonpolar or a glass tube composed of bonds Why?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


