Question
Question 1 Alum (Alz (SO4)3 14H2O) is being used in a coagulation process to form precipitate for treating water. A stock solution of alum
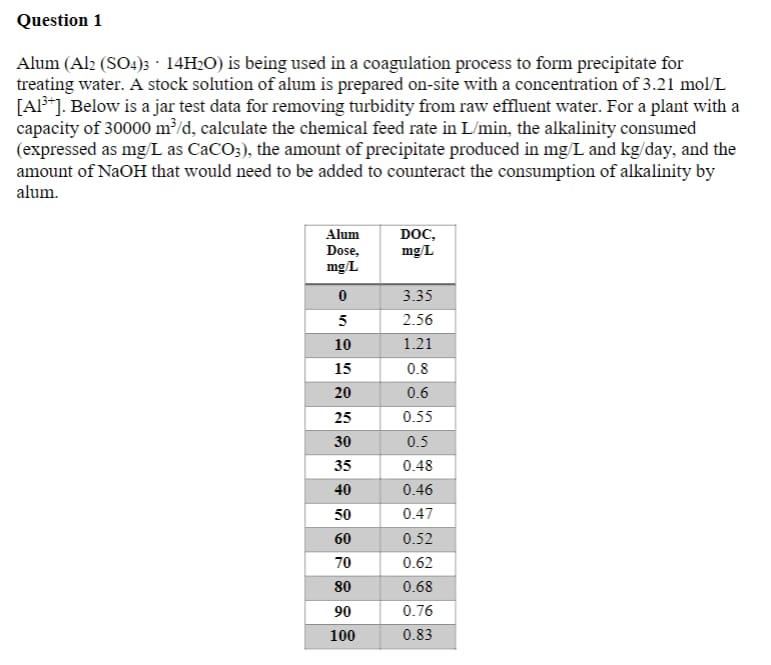
Question 1 Alum (Alz (SO4)3 14H2O) is being used in a coagulation process to form precipitate for treating water. A stock solution of alum is prepared on-site with a concentration of 3.21 mol/L [AP]. Below is a jar test data for removing turbidity from raw effluent water. For a plant with a capacity of 30000 m/d, calculate the chemical feed rate in L/min, the alkalinity consumed (expressed as mg L as CaCO;), the amount of precipitate produced in mg/L and kg/day, and the amount of NaOH that would need to be added to counteract the consumption of alkalinity by alum. DOC, mg/L Alum Dose, mg/L 3.35 5 2.56 10 1.21 15 0.8 20 0.6 25 0.55 30 0.5 35 0.48 40 0.46 50 0.47 60 0.52 70 0.62 80 0.68 90 0.76 100 0.83
Step by Step Solution
3.41 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Consumer Behavior Buying, Having and Being
Authors: Michael R. Solomon
11th edition
978-0133451153, 133450899, 133451151, 978-0133450897
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



