Answered step by step
Verified Expert Solution
Question
1 Approved Answer
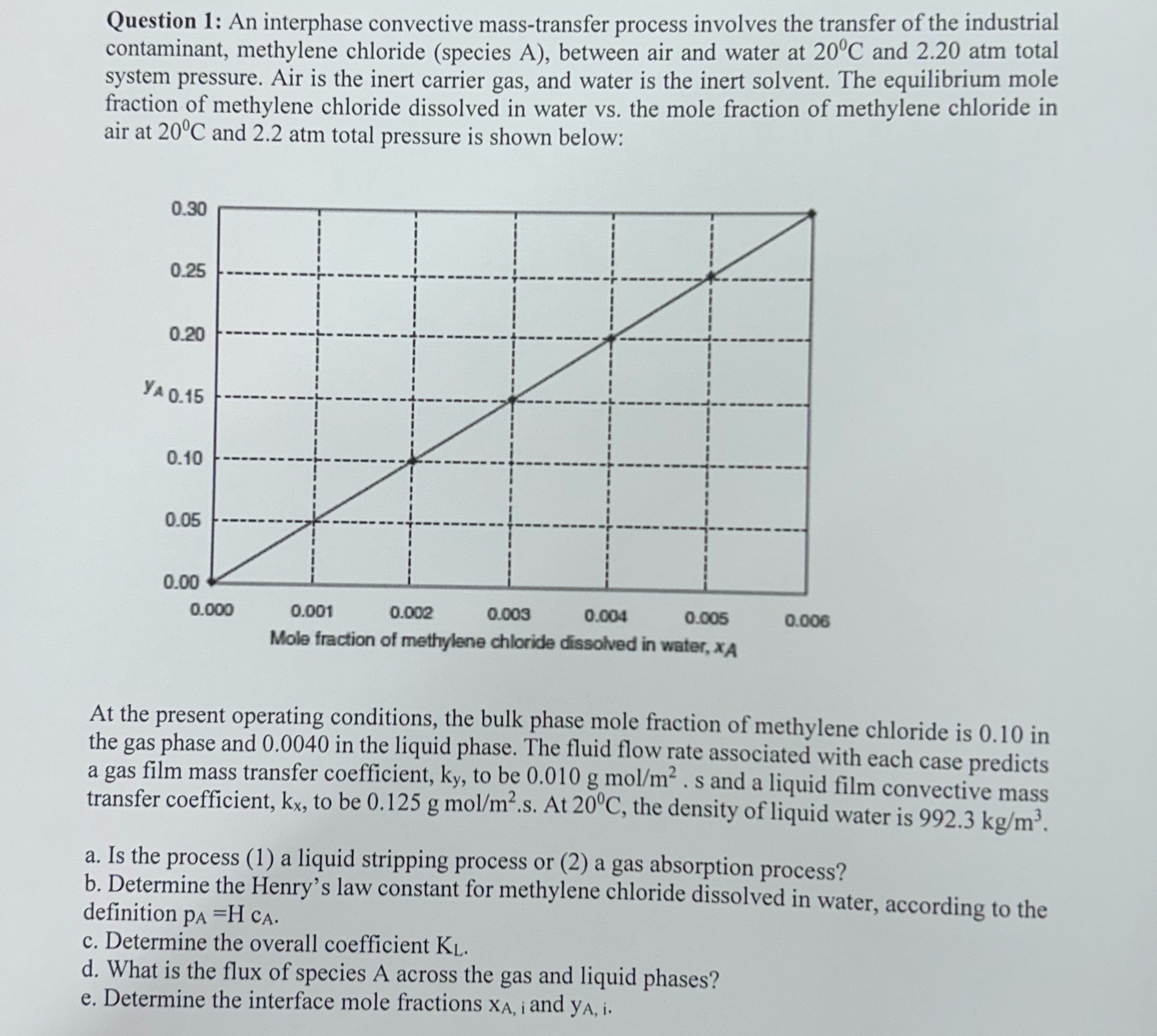
Question 1 : An interphase convective mass - transfer process involves the transfer of the industrial contaminant, methylene chloride ( species A ) , between
Question : An interphase convective masstransfer process involves the transfer of the industrial contaminant, methylene chloride species A between air and water at and atm total system pressure. Air is the inert carrier gas, and water is the inert solvent. The equilibrium mole fraction of methylene chloride dissolved in water vs the mole fraction of methylene chloride in air at and atm total pressure is shown below:
At the present operating conditions, the bulk phase mole fraction of methylene chloride is in the gas phase and in the liquid phase. The fluid flow rate associated with each case predicts a gas film mass transfer coefficient, to be gmo and a liquid film convective mass transfer coefficient, to be gmo At the density of liquid water is
a Is the process a liquid stripping process or a gas absorption process?
b Determine the Henry's law constant for methylene chloride dissolved in water, according to the definition
c Determine the overall coefficient
d What is the flux of species A across the gas and liquid phases?
e Determine the interface mole fractions and
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


