Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question: 1. Calculate the molar extinction coefficient and provide the value and the appropriate unit. 2. Comment on the value of you have calculated. Do



Question:
1. Calculate the molar extinction coefficient and provide the value and the appropriate unit.
2. Comment on the value of you have calculated. Do you think the colour in this complex arises from dd transitions? Does your answer make sense considering the coordination geometry at the irons?
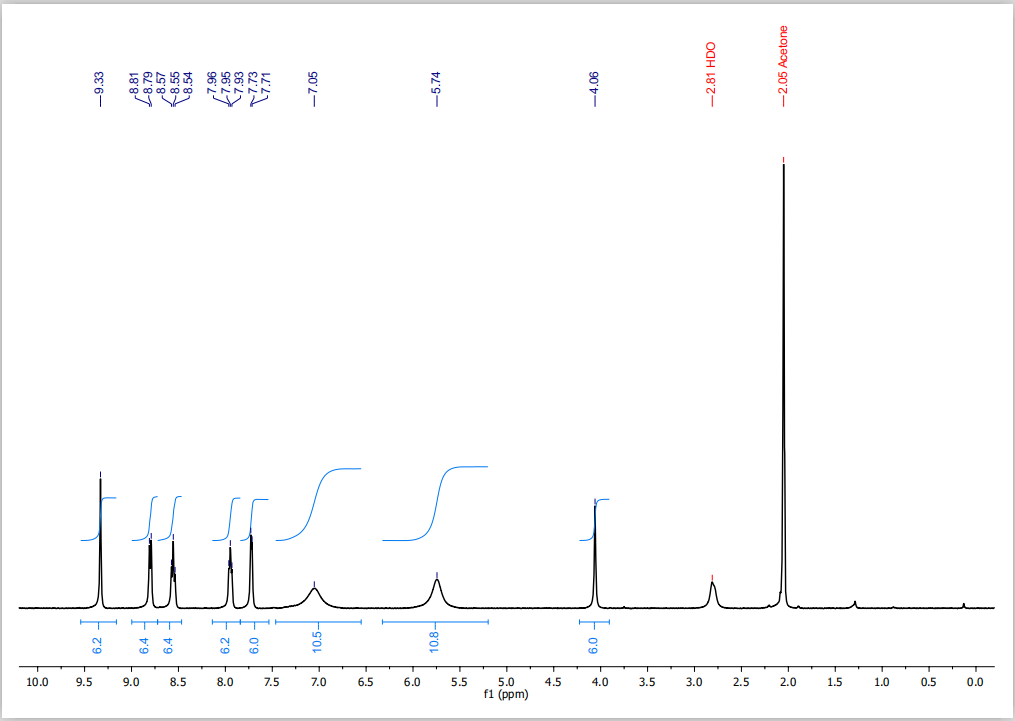
Synthesis of [Fe2L3](PF6)4 (Individual activity) Dissolve 0. 120 g of 4,4'-methylenedianiline in 20 mL methanol. Dissolve 0.080 g of iron(II) chloride tetrahydrate in 10 mL methanol. Add the iron solution to the solution of 4,4'-methylenedianiline and then add 124 uL of 2-pyridinecarboxaldehyde using a micropipette. Stir the resulting solution for 15 minutes. After 15 minutes, add a solution of 163 mg ammonium hexafluorophosphate in 20 mL methanol to your reaction mixture. This should result in precipitation of a solid. Filter this solid, wash with 2 x 10 mL methanol, and dry by suction. Record the mass of your product. Dispose of the filtrate from your synthesis in the appropriate waste bottle. UVNis Spectroscopy Prepare a 1.0 * 10-3 mol L-1 stock solutions of your complex in acetone in a 10 mL volumetric flask (Hint: the molar mass of your product is 1821 g mol-1). Use a disposable pipette to make it up to the line and then mix by multiple inversions. Dilute this by a factor of 50 to give you a 2.0 x 10-5 mol L-1 solution: i.e. add 0.20 mL of your stock solution to a 10 mL volumetric flask and then make up to the line with more acetone. Mix by multiple inversions. Record the UVNis spectrum of this 2.0 x 10-5 mol L-1 solution from 350650 nm in glass cuvettes. Molecular Models Ball and stick models have been provided on the bench. Your product forms as a mixture of two isomers with the formula [Fe2L3) (PF6). Build the models of the two isomers from the structural components provided and take photos to attach to your lab report. When you are done, please deconstruct your model (back to the starting materials), so the next person can use it. Paper chromatography of [Fe2L3]Cl4 (Demonstration activity) This experiment has been carried out by the demonstrator ensure you observe the results before leaving the laboratory. [Fe2L3](PF6)4 (the complex you synthesize) has been converted to the chloride salt using an excess of anion exchange resin in the chloride form. Two stripes of Whatman chromatography paper (o-cellulose) that are about 2.5 cm wide and 14 cm long have been cut. The solution was spotted multiple times (until there is a large spot about 0.5 cm in diameter) 1.5 cm from the bottom of one of the paper strips using a capillary tube. The paper is allowed to briefly dry in air. The second piece of paper was attached to the top of the first with a paper clip. The chromatograph is developed by carefully placing in a tall tank containing 20 mM NaCl(aq) (less than 1 cm in depth inside the chamber). The end of the paper is hooked over the edge of the tank in so that the paper does not slump down when it gets wet. The eluant will be drawn up the paper by capillary action and you should see a separation of the compound into two spots. Yield = 0.275 g Colour and appearance: dark purple powder UV-vis spectrum results: Helicate at a concentration of 2.0 x 10-5 mol L in a 1 cm cell- Amax = 572 nm and absorbance at peak = 0.276 Abs- = -9.33 -2.05 Acetone -7.05 -5.74 -2.81 HDO -4.06 6.2 co o CO 10.0 9.5 9.0 8.5 8.0 7.5 7.0 6.5 6.0 5.5 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 0.5 0.0 5.0 f1 (ppm)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


