Answered step by step
Verified Expert Solution
Question
1 Approved Answer
QUESTION 1 Glve short answers to each of the following questions. Do not copy the questions. Just give the answer only Make sure that your
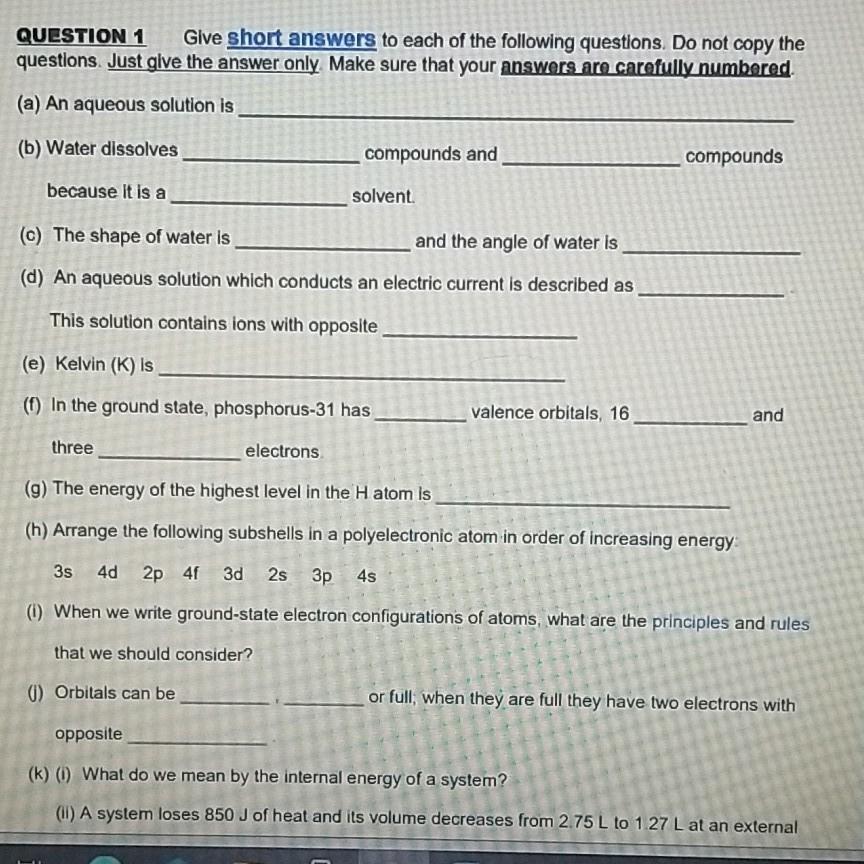
QUESTION 1 Glve short answers to each of the following questions. Do not copy the questions. Just give the answer only Make sure that your answers are carefully numbered (a) An aqueous solution is (b) Water dissolves compounds and compounds because it is a solvent (c) The shape of water is and the angle of water is (d) An aqueous solution which conducts an electric current is described as This solution contains ions with opposite (e) Kelvin (K) Is () In the ground state, phosphorus-31 has valence orbitals, 16 and three electrons (9) The energy of the highest level in the H atom is (h) Arrange the following subshells in a polyelectronic atom in order of increasing energy 3s 4d 2p 41 3d 2s 3p4s ( When we write ground-state electron configurations of atoms, what are the principles and rules that we should consider? () Orbitals can be or full, when they are full they have two electrons with opposite (k) 0 What do we mean by the internal energy of a system? (II) A system loses 850 J of heat and its volume decreases from 2.75 L to 1 27 L at an external
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


