Question
Question 1 ) In a catalytic reactor, cylindrically shaped particles, 1 cm long and with a radius = 0.3 cm, are surrounded by a gaseous
Question 1 ) In a catalytic reactor, cylindrically shaped particles, 1 cm long and with a radius = 0.3 cm, are surrounded by a gaseous layer, 0.2 cm thick, through which the feed of a mixture A and B, containing 50% of A, diffuses and reacts at the surface. The reaction takes place according to the simplified equation
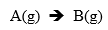
The rate of the reaction at the surface with respect to component A(g) is described by: NA = -KsCA .The system is at 300C and 1 atm, where Ks = 2.0 cm/s and DA-gaseous mixture => DA-M = 0.108 cm2/s.
a ) The diffusion rate (WA) in mols/s .
b) What considerations make the homogeneous system approach different from the heterogeneous system approach?
c)Also in relation to the previous problem : If it were limited by mass transport, how could we minimize this resistance? and If it were limited by chemical kinetics, how could we minimize this resistance?
A(g) B(g)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


