Answered step by step
Verified Expert Solution
Question
1 Approved Answer
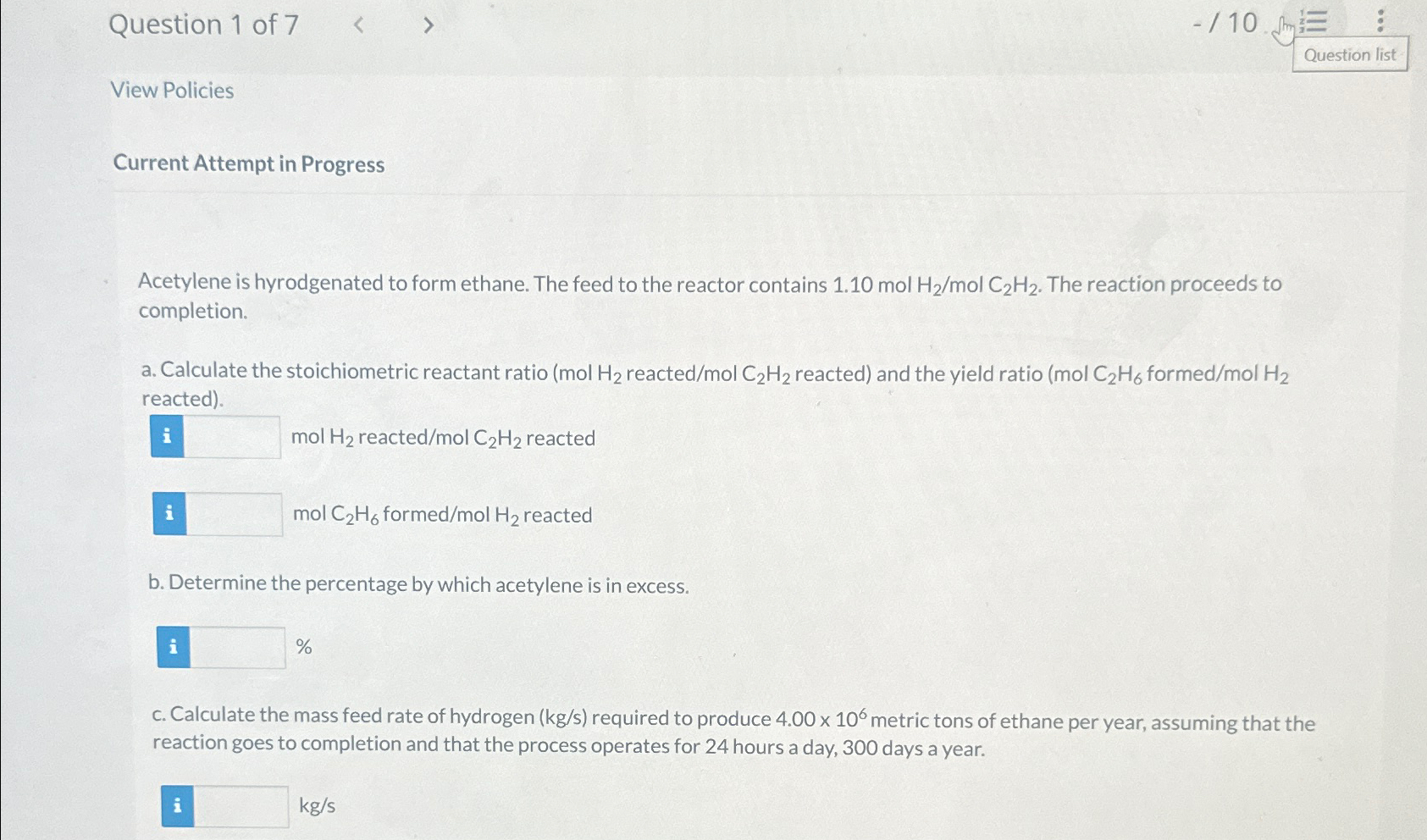
Question 1 of 7 View Policies Current Attempt in Progress Acetylene is hyrodgenated to form ethane. The feed to the reactor contains 1 . 1
Question of
View Policies
Current Attempt in Progress
Acetylene is hyrodgenated to form ethane. The feed to the reactor contains mol The reaction proceeds to completion.
a Calculate the stoichiometric reactant ratio reacted reacted and the yield ratio formed reacted
mol reacted reacted
mol formed reacted
b Determine the percentage by which acetylene is in excess.
c Calculate the mass feed rate of hydrogen required to produce metric tons of ethane per year, assuming that the reaction goes to completion and that the process operates for hours a day, days a year.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


