Answered step by step
Verified Expert Solution
Question
1 Approved Answer
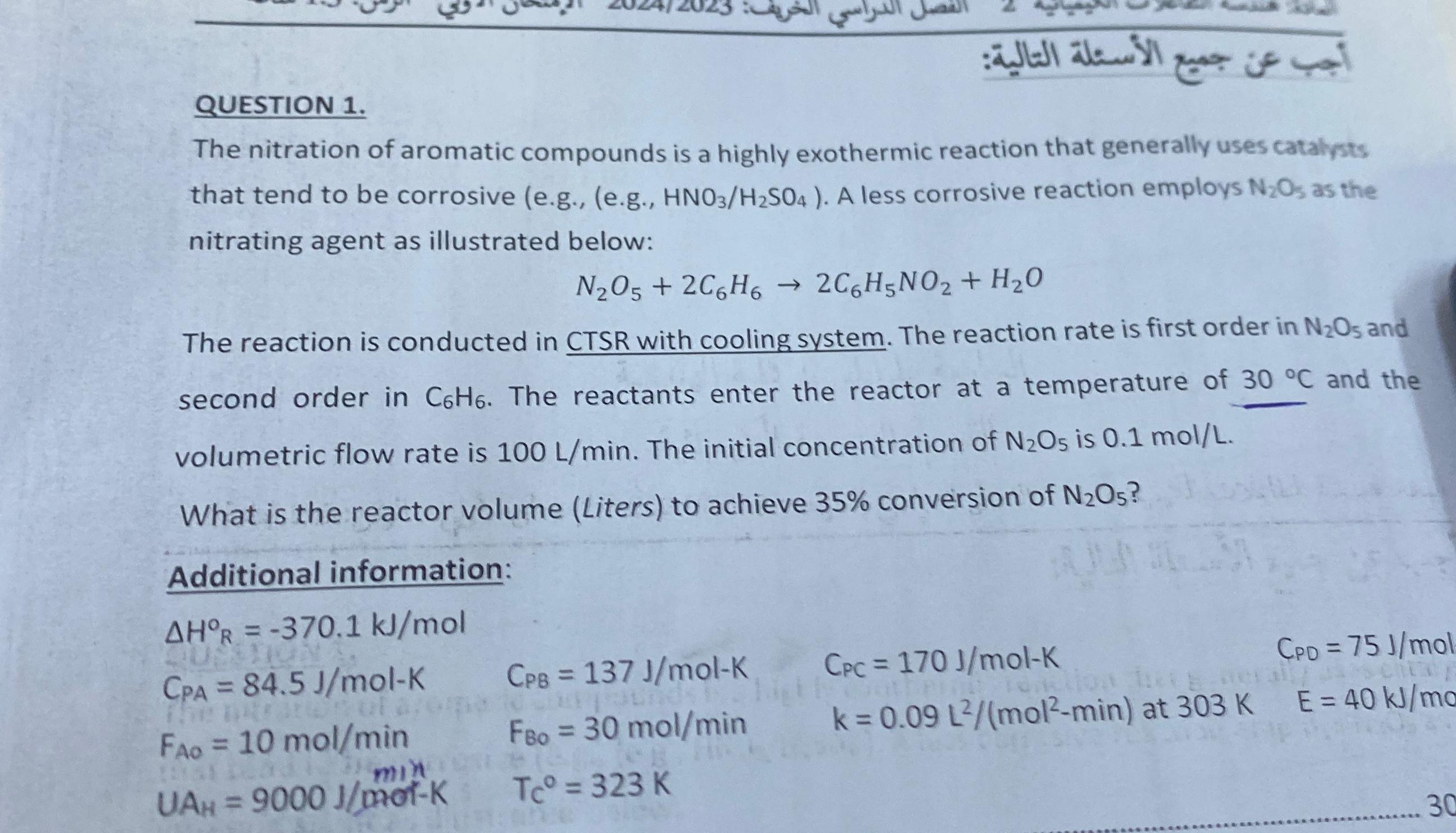
QUESTION 1 . : The nitration of aromatic compounds is a highly exothermic reaction that generally uses catalysts that tend to be corrosive ( e
QUESTION
:
The nitration of aromatic compounds is a highly exothermic reaction that generally uses catalysts that tend to be corrosive egeg A less corrosive reaction employs as the nitrating agent as illustrated below:
The reaction is conducted in CTSR with cooling system. The reaction rate is first order in and second order in The reactants enter the reactor at a temperature of and the volumetric flow rate is The initial concentration of is
What is the reactor volume Liters to achieve conversion of
Additional information:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


