Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 1: You discover some polymer in a bottle in the process of cleaning out the Lab's 80C freezer and are tasked with identifying it.
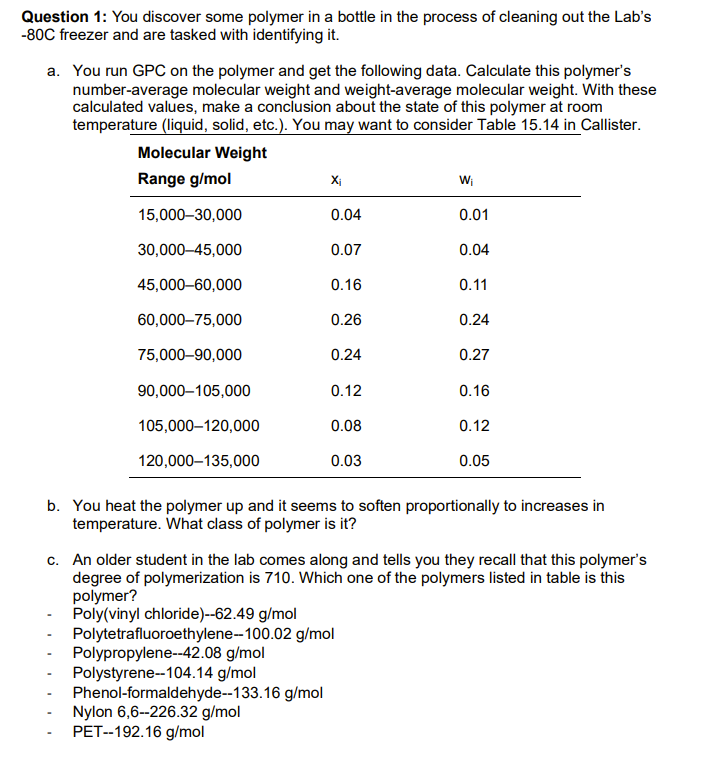 Question 1: You discover some polymer in a bottle in the process of cleaning out the Lab's 80C freezer and are tasked with identifying it. a. You run GPC on the polymer and get the following data. Calculate this polymer's number-average molecular weight and weight-average molecular weight. With these calculated values, make a conclusion about the state of this polymer at room temperature (liquid, solid, etc.). You may want to consider Table 15.14 in Callister. b. You heat the polymer up and it seems to soften proportionally to increases in temperature. What class of polymer is it? c. An older student in the lab comes along and tells you they recall that this polymer's degree of polymerization is 710 . Which one of the polymers listed in table is this polymer? - Poly(vinyl chloride)--62.49 g/mol - Polytetrafluoroethylene--100.02 g/mol - Polypropylene--42.08 g/mol - Polystyrene--104.14 g/mol - Phenol-formaldehyde--133.16 g/mol - Nylon 6,6--226.32 g/mol - PET--192.16 g/mol
Question 1: You discover some polymer in a bottle in the process of cleaning out the Lab's 80C freezer and are tasked with identifying it. a. You run GPC on the polymer and get the following data. Calculate this polymer's number-average molecular weight and weight-average molecular weight. With these calculated values, make a conclusion about the state of this polymer at room temperature (liquid, solid, etc.). You may want to consider Table 15.14 in Callister. b. You heat the polymer up and it seems to soften proportionally to increases in temperature. What class of polymer is it? c. An older student in the lab comes along and tells you they recall that this polymer's degree of polymerization is 710 . Which one of the polymers listed in table is this polymer? - Poly(vinyl chloride)--62.49 g/mol - Polytetrafluoroethylene--100.02 g/mol - Polypropylene--42.08 g/mol - Polystyrene--104.14 g/mol - Phenol-formaldehyde--133.16 g/mol - Nylon 6,6--226.32 g/mol - PET--192.16 g/mol Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


