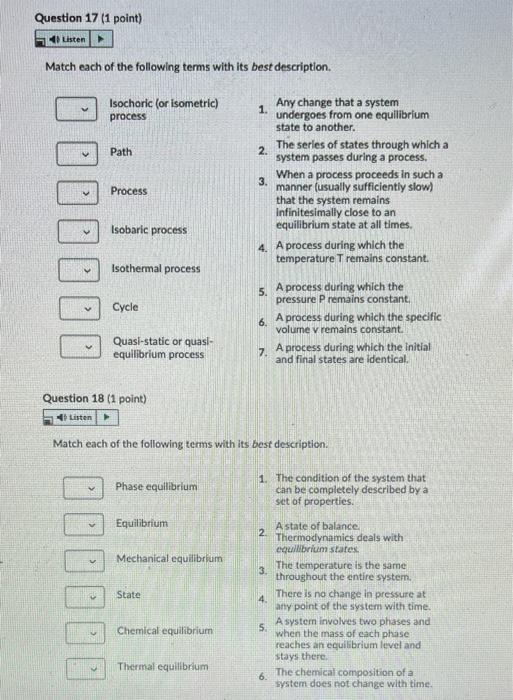
Question 15 (1 point) Listen Matching each of the terms below with its best description Property 1. Any characteristic of a system, either intensive or extensive. Extensive properties Intensive properties 2. Properties that are independent of the mass of a system, such as temperature, pressure, and density. 3 Properties whose values depend on the size - or extent - of the system. 4. Extensive properties per unit mass. Specific properties Question 16 (1 point) Listen > Match each of the following terms with its definition . mass per unit volume, 1. p= (kg/m) 2. volume per unit mass, reciprocal of density = } (m/kg) specific gravity - density 3. specific weight weight per unit volume, Ys = pg (N/m?) 4. also known as relative density, the ratio of the density of a substance to the density of some standard substance at a specified temperature (usually water at 4C). SG = dimensionless. Pito specific volume Question 17 (1 point) Listen Match each of the following terms with its best description. Isochoric (or isometric) process Path Any change that a system 1. undergoes from one equilibrium state to another. 2. The series of states through which a system passes during a process, When a process proceeds in such a 3. manner (usually sufficiently slow) that the system remains Infinitesimally close to an equilibrium state at all times. 4. A process during which the temperature Tremains constant. Process Isobaric process Isothermal process 5. Cycle 6. A process during which the pressure P remains constant, A process during which the specific volume v remains constant A process during which the initial and final states are identical. Quasi-static or quasi- equilibrium process 7. Question 18 (1 point) Listen Match each of the following terms with its best description. Phase equilibrium 1. The condition of the system that can be completely described by a set of properties. Equilibrium 2 Mechanical equilibrium 3. DIDDOP State 4. A state of balance Thermodynamics deals with equilibrium states The temperature is the same throughout the entire system. There is no change in pressure at any point of the system with time. A system involves two phases and when the mass of each phase reaches an equilibrium level and Stays there The chemical composition of a system does not change with time. Chemical equilibrium 5. Thermal equilibrium 6