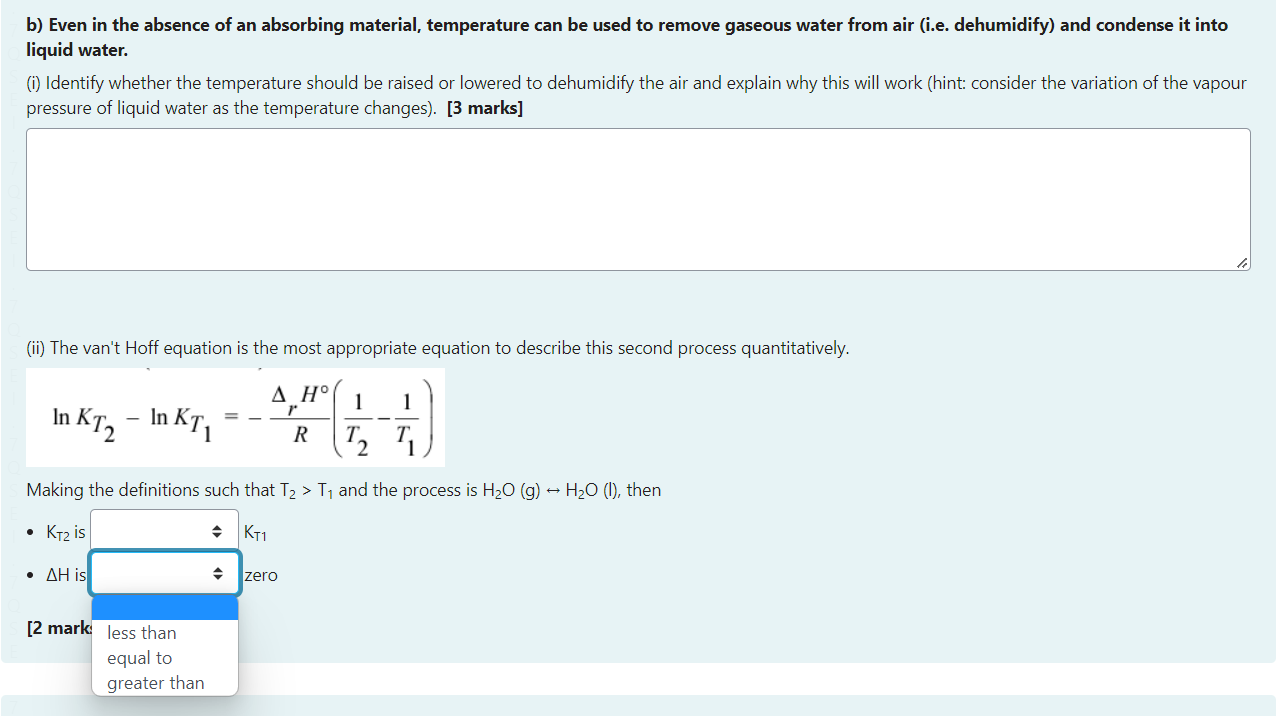
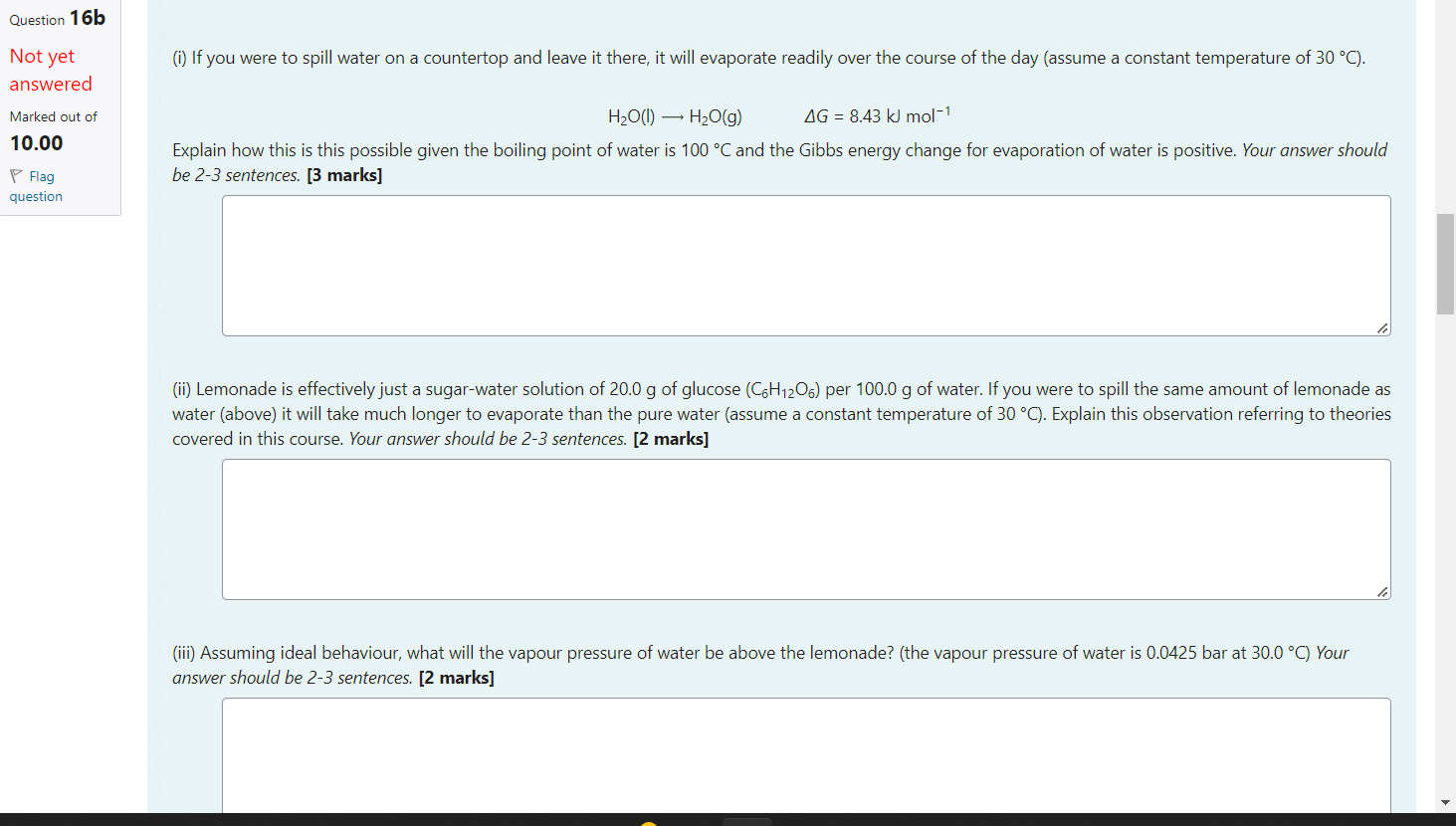
Question 16a a) Some food packages contain small packets designed to absorb water and so keep the food fresh for longer. Not yet (i) Describe the chemistry (including the intermolecular forces) involved in this absorption process and thus identify the chemical properties required of this answered material (e.g. what functional groups or bond types). [3 marks] Marked out of 10.00 P Flag question (ii) These packets can sometimes be reused after warming then to remove the water they have absorbed. We can explain this phenomenon using your understanding of equilibrium chemistry by doing these two things: 1. Write a chemical reaction to describe this process (You can use > to indicate equilibrium arrows). 2. Describe what happens to the equilibrium as the temperature increases and thus conclude whether increased temperature leads to a decrease or increase or constant equilibrium constant. b) Even in the absence of an absorbing material, temperature can be used to remove gaseous water from air (i.e. dehumidify) and condense it into liquid water. (i) Identify whether the temperature should be raised or lowered to dehumidify the air and explain why this will work (hint: consider the variation of the vapour pressure of liquid water as the temperature changes). [3 marks] (ii) The van't Hoff equation is the most appropriate equation to describe this second process quantitatively. lnKT2lnKT1=RrH(T21T11) H2O(l)H2O(g)G=8.43kJmol1 Explain how this is this possible given the boiling point of water is 100C and the Gibbs energy change for evaporation of water is positive. Your answer should be 2-3 sentences. [3 marks] (ii) Lemonade is effectively just a sugar-water solution of 20.0g of glucose (C6H12O6) per 100.0g of water. If you were to spill the same amount of lemonade as water (above) it will take much longer to evaporate than the pure water (assume a constant temperature of 30C ). Explain this observation referring to theories covered in this course. Your answer should be 2-3 sentences. [2 marks] (iii) Assuming ideal behaviour, what will the vapour pressure of water be above the lemonade? (the vapour pressure of water is 0.0425 bar at 30.0C ) Your answer should be 2-3 sentences. [2 marks]









