Answered step by step
Verified Expert Solution
Question
1 Approved Answer
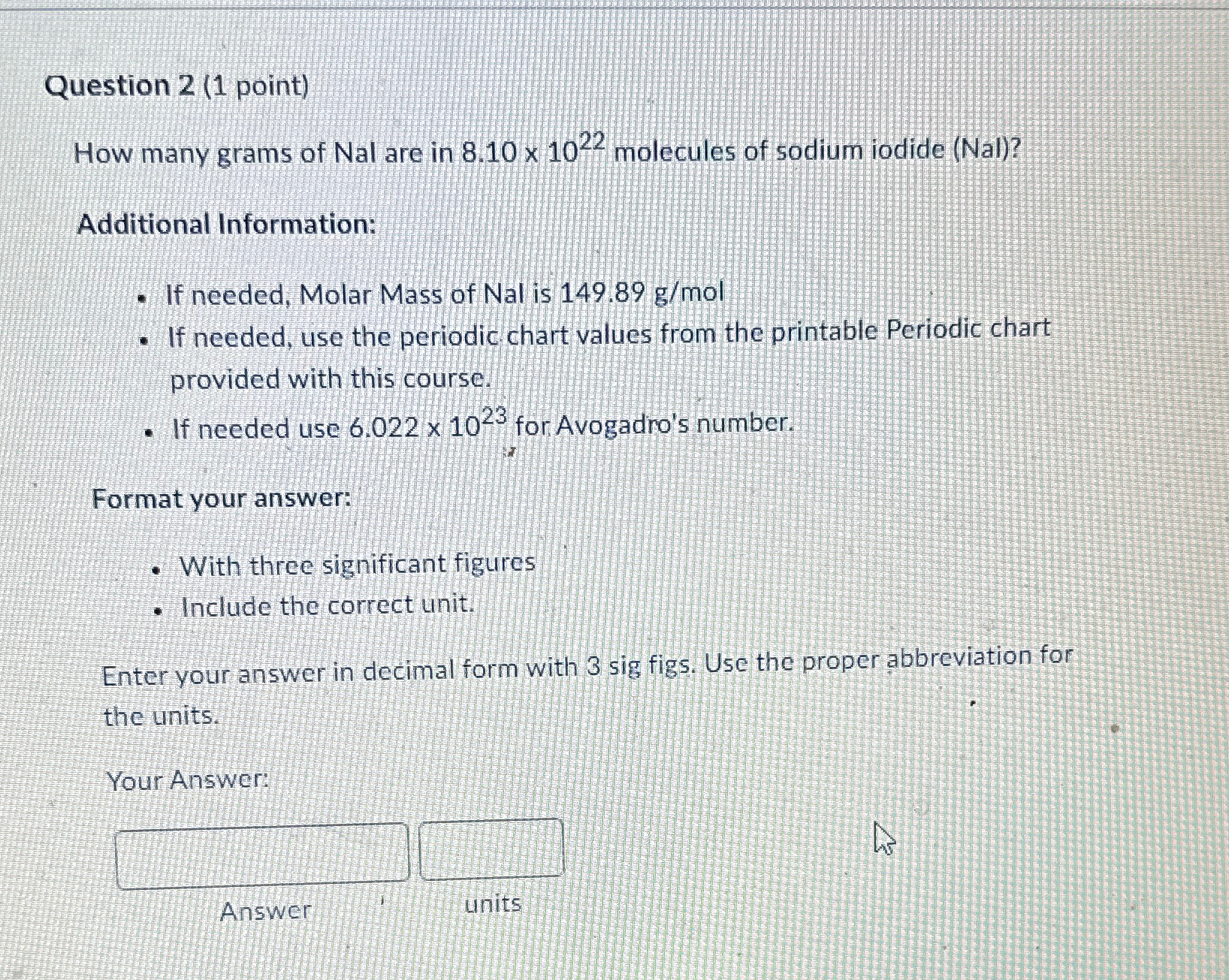
Question 2 (1 point) How many grams of Nal are in 8.10times 10^(22) molecules of sodium iodide (Nal)? Additional Information: If needed, Molar Mass of
Question 2 (1 point)\ How many grams of
Nalare in
8.10\\\\times 10^(22)molecules of sodium iodide (Nal)?\ Additional Information:\ If needed, Molar Mass of Nal is
149.89(g)/(m)ol\ If needed, use the periodic chart values from the printable Periodic chart provided with this course.\ If needed use
6.022\\\\times 10^(23)for Avogadro's number.\ Format your answer:\ With three significant figures\ Include the correct unit.\ Enter your answer in decimal form with 3 sig figs. Use the proper abbreviation for the units.\ Your Answer:\ Answer\ units
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


