Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 2 ( 2 0 pts ) . Ethane is mixed with oxygen gas which is coming from a storage tank in which the temperature
Question pts Ethane is mixed with oxygen gas which is coming from a storage tank in which the temperature is before this mixture is sent to a combustion chamber. The volumetric flowrate of the oxygen gas which is coming from the storage tank is mhr In the combustion chamber the ethaneoxygen mixture is burned with excess air and the exhaust gas from the chamber has a volumetric flowrate of mhr at standard conditions. The composition of the exhaust gas is mole O mole N mole CO mole CO and mole HO Calculate the pressure kPa inside the oxygen gas storage tank by using the van der Waals equation of state.
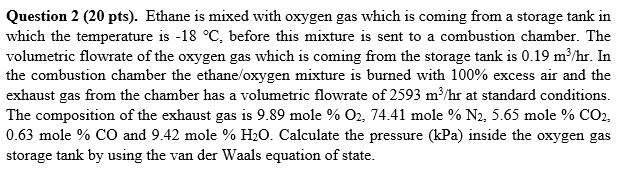
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


