Answered step by step
Verified Expert Solution
Question
1 Approved Answer
QUESTION 2 : ( 2 0 pts ) Thermodynamics of polymer solutions ( i ) Write down the Generalized Flory - Huggins Equation ( GFHE
QUESTION : pts Thermodynamics of polymer solutions
i Write down the Generalized FloryHuggins Equation GFHE for binary mixtures,
A and Bper site Define each symbol you use and identify clearly the enthalpy
and the entropy terms.
ii Using GFHE above, find the critical point in terms of and Then,
determine the solubility criteria the mixture of two small molecules and for the
mixture of polymer and a solvent.
iii Now repeat part ii for a symmetric blend of two polymer in the limit of
infinite molecular weight. Compare your result with those from ii and discuss the
solubility criteria for this case.
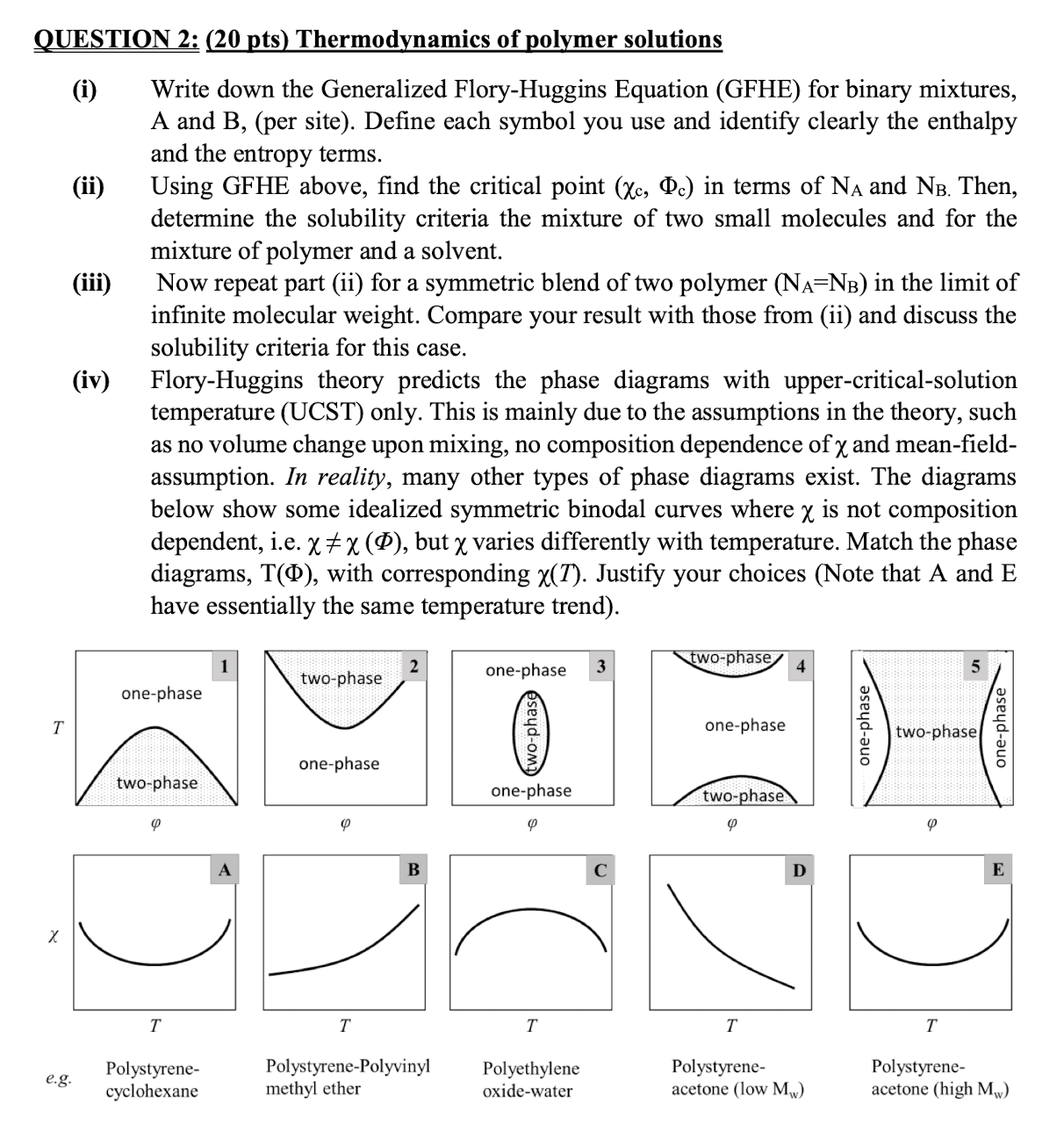
iv FloryHuggins theory predicts the phase diagrams with uppercriticalsolution
temperature UCST only. This is mainly due to the assumptions in the theory, such
as no volume change upon mixing, no composition dependence of and meanfield
assumption. In reality, many other types of phase diagrams exist. The diagrams
below show some idealized symmetric binodal curves where is not composition
dependent, ie but varies differently with temperature. Match the phase
diagrams, with corresponding Justify your choices Note that A and E
have essentially the same temperature trend
T
T
PolystyrenePolyvinyl
methyl ether
T
Polyethylene
oxidewater
Polystyrene
acetone low
Polystyrene
acetone high Thermodynamics of polymer solutions
i Write down the Generalized FloryHuggins Equation GFHE for binary mixtures,
A and Bper site Define each symbol you use and identify clearly the enthalpy and the entropy terms.
ii Using GFHE above, find the critical point chi cPhi c in terms of NA and NB Then,
determine the solubility criteria the mixture of two small molecules and for the mixture of polymer and a solvent. iii Now repeat part ii for a symmetric blend of two polymer NANB in the limit of
infinite molecular weight. Compare your result with those from ii and discuss the solubility criteria for this case. iv FloryHuggins theory predicts the phase diagrams with uppercriticalsolution
temperature UCST only. This is mainly due to the assumptions in the theory, such as no volume change upon mixing, no composition dependence of chi and meanfield assumption. In reality, many other types of phase diagrams exist. The diagrams below show some idealized symmetric binodal curves where chi is not composition dependent, iechi chi Phi but chi varies differently with temperature. Match the phase diagrams, TPhi with corresponding chi T Justify your choices Note that A and E have essentially the same temperature trend
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


