Question
Question 2 a). Evaluate effectiveness factor (h) for a first order irreversible reaction at three values of Thiele's modulus (f) in the range f-
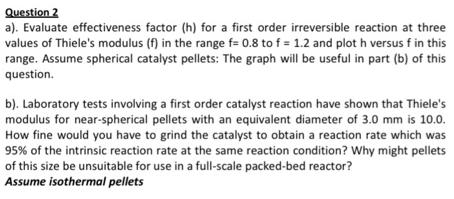
Question 2 a). Evaluate effectiveness factor (h) for a first order irreversible reaction at three values of Thiele's modulus (f) in the range f- 0.8 to f 1.2 and plot h versus f in this range. Assume spherical catalyst pellets: The graph will be useful in part (b) of this question b). Laboratory tests involving a first order catalyst reaction have shown that Thiele's modulus for near-spherical pellets with an equivalent diameter of 3.0 mm is 10.0. How fine would you have to grind the catalyst to obtain a reaction rate which was 95% of the intrinsic reaction rate at the same reaction condition? Why might pellets of this size be unsuitable for use in a full-scale packed-bed reactor? Assume isothermal pellets
Step by Step Solution
3.59 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Finite Mathematics and Its Applications
Authors: Larry J. Goldstein, David I. Schneider, Martha J. Siegel, Steven Hair
12th edition
978-0134768588, 9780134437767, 134768582, 134437764, 978-0134768632
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



