Answered step by step
Verified Expert Solution
Question
1 Approved Answer
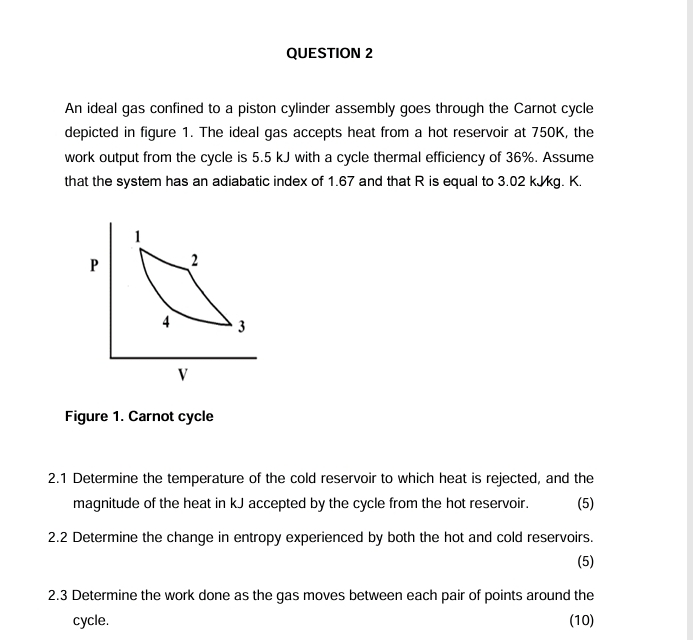
QUESTION 2 An ideal gas confined to a piston cylinder assembly goes through the Carnot cycle depicted in figure 1 . The ideal gas accepts
QUESTION
An ideal gas confined to a piston cylinder assembly goes through the Carnot cycle depicted in figure The ideal gas accepts heat from a hot reservoir at K the work output from the cycle is kJ with a cycle thermal efficiency of Assume that the system has an adiabatic index of and that is equal to K
Figure Carnot cycle
Determine the temperature of the cold reservoir to which heat is rejected, and the magnitude of the heat in kJ accepted by the cycle from the hot reservoir.
Determine the change in entropy experienced by both the hot and cold reservoirs.
Determine the work done as the gas moves between each pair of points around the cycle.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


