Question
Question 2: Data analysis and reactor design The stoichiometry of a non-elementary liquid-phase reaction can be represented by ERT k- A e the reaction:
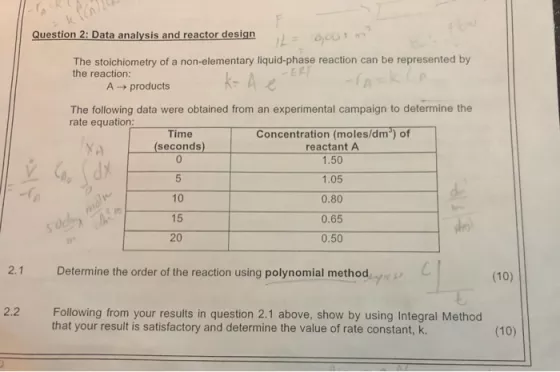
Question 2: Data analysis and reactor design The stoichiometry of a non-elementary liquid-phase reaction can be represented by ERT k- A e the reaction: A- products The following data were obtained from an experimental campaign to determine the rate equation: Concentration (moles/dm') of reactant A Time (seconds) 1.50 1.05 XP 10 0.80 15 0.65 20 0.50 2.1 Determine the order of the reaction using polynomial method (10) 2.2 Following from your results in question 2.1 above, show by using Integral Method that your result is satisfactory and determine the value of rate constant, k. (10)
Step by Step Solution
3.48 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
of reacton 21 Determine the orde reve of ois app...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Numerical Analysis
Authors: Richard L. Burden, J. Douglas Faires
9th edition
538733519, 978-1133169338, 1133169333, 978-0538733519
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



