Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 2: Diffusion plus convection with chemical reaction Pure gas A diffuses from the bulk gas (point 1) to a catalyst surface (point 2) where
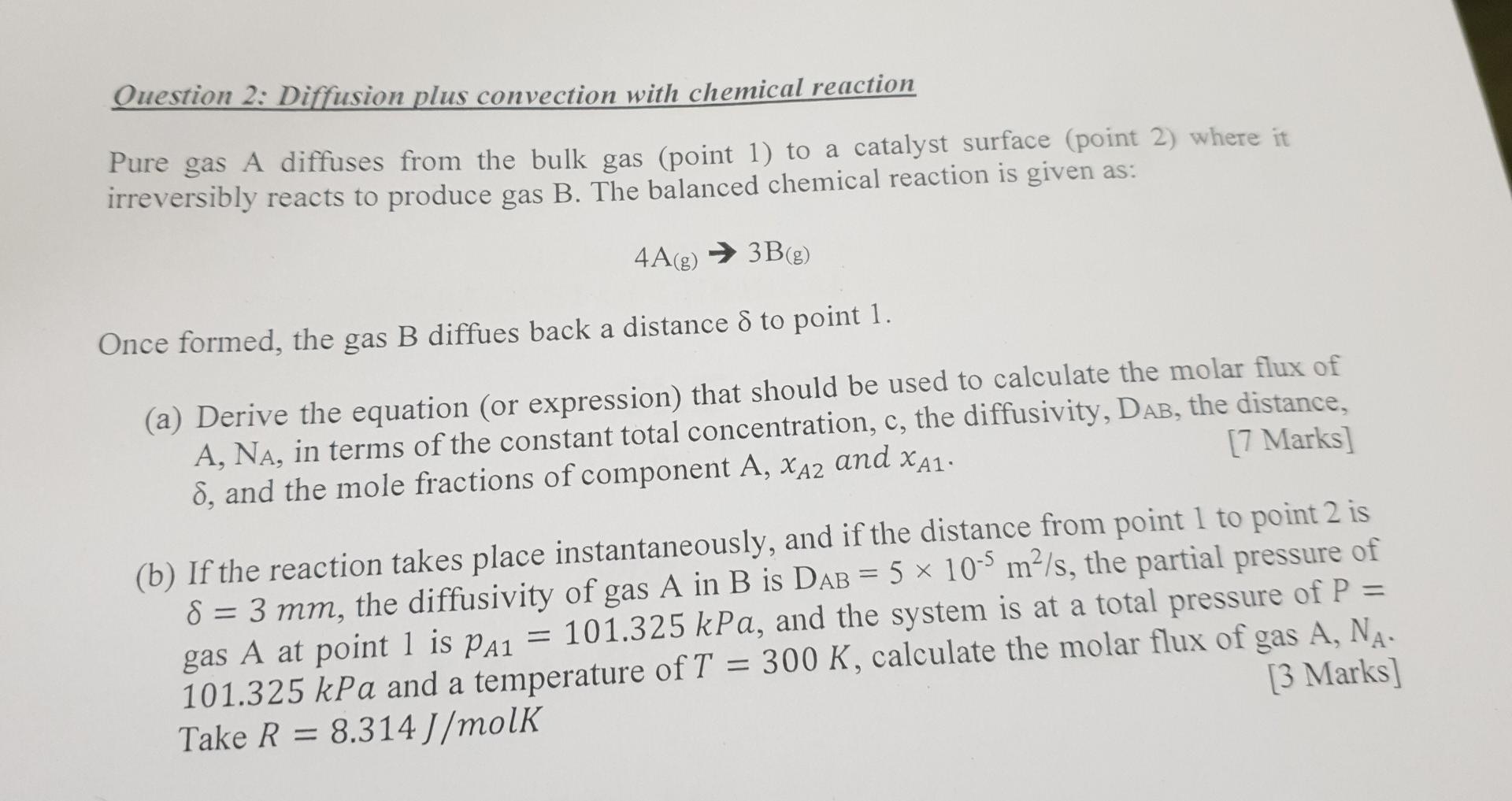
Question 2: Diffusion plus convection with chemical reaction Pure gas A diffuses from the bulk gas (point 1) to a catalyst surface (point 2) where it irreversibly reacts to produce gas B. The balanced chemical reaction is given as: 4A(g) 3B(g) Once formed, the gas B diffues back a distance S to point 1. (a) Derive the equation (or expression) that should be used to calculate the molar flux of A, NA, in terms of the constant total concentration, c, the diffusivity, DAB, the distance, d, and the mole fractions of component A, XA2 and XA1. [7 Marks] = (b) If the reaction takes place instantaneously, and if the distance from point 1 to point 2 is 8 = 3 mm, the diffusivity of gas A in B is DAB = 5 x 10 m/s, the partial pressure of gas A at point 1 is pai = 101.325 kPa, and the system is at a total pressure of P = 101.325 kPa and a temperature of T = 300 K, calculate the molar flux of gas A, NA- Take R = 8.314 J/molk [3 Marks] =
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


