Question
How many atoms of 102.91 grams of Rh? 19 b) 6.022x10 23 d) 7.301x1012 e) none of the answers a) 9.119 x10 c) 7.301
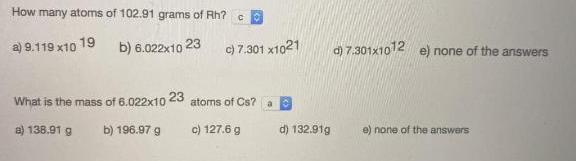
How many atoms of 102.91 grams of Rh? 19 b) 6.022x10 23 d) 7.301x1012 e) none of the answers a) 9.119 x10 c) 7.301 x1021 What is the mass of 6.022x10 23 atoms of Cs? a) 138.91 g b) 196.97 g c) 127.6 g d) 132.91g e) none of the answers
Step by Step Solution
3.34 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Conceptual Physical Science
Authors: Paul G. Hewitt, John A. Suchocki, Leslie A. Hewitt
6th edition
013408229X, 978-0134082295, 9780134080512 , 978-0134060491
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



