Question
Question #2 The kinetics of the gas-phase reaction between nitrogen dioxide, NOz (A) and trichloroethene (B) have been investigated over the range 303-362 2 K.
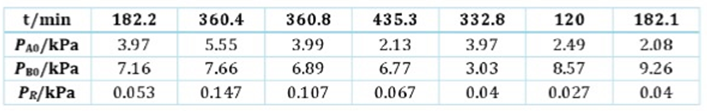
Question #2 The kinetics of the gas-phase reaction between nitrogen dioxide, NOz (A) and trichloroethene (B) have been investigated over the range 303-362 2 K. The reaction extent, with the reaction carried out ina constant-volume batch reactor, was determined from measurements of infrared absorption intensities, which were converted into corresponding pressures by calibration. The products of the reaction are nitrosyl chloride NOCI, (R), and glyoxyloxyl chloride(S) In a series of seven experiments at 323.1 K, the initial pressures, PAo and PBo Were varied, and the partial pressure of NOC, PRwas measured after a certain length of time. Results are as follows:
a. Write the chemical equation representing the stoichiometry of the reaction
b. Can the course of the reaction be followed by measuring (total) pressure rather than by the method described above? Explain.
c. Determine the form of the rate law and the value of the rate constant (in units of L mol s)at 323.1 K. with respect to NO 2 (A). Derive all necessary equations
t/min 182.2 360.4 360.8 Ps/kPa 3.97 5.55 3.99 P/ 7.16 7.66 6.89 PR/kPa 0.053 0.147 0.107 435.3 2.13 6.77 0.067 332.8 3.97 3.03 0.04 120 2.49 8.57 0.027 182.1 2.08 9.26 0.04
Step by Step Solution
3.39 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
a 2NO 2 C 2 HCl 3 2NOCl C 2 HClO 2 b No I dont think deter...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


