Answered step by step
Verified Expert Solution
Question
1 Approved Answer
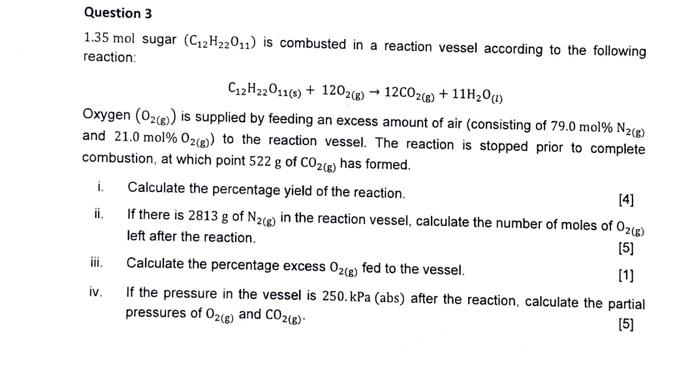
Question 3 1.35 mol sugar (C2H22011) is combusted in a reaction vessel according to the following reaction: C12H22011(s) + 1202(g) 12CO2(g) + 11H0 (1) Oxygen
Question 3 1.35 mol sugar (C2H22011) is combusted in a reaction vessel according to the following reaction: C12H22011(s) + 1202(g) 12CO2(g) + 11H0 (1) Oxygen (O2(g)) is supplied by feeding an excess amount of air (consisting of 79.0 mol % N(g) and 21.0 mol % O2(g)) to the reaction vessel. The reaction is stopped prior to complete combustion, at which point 522 g of CO2(g) has formed. Calculate the percentage yield of the reaction. [4] If there is 2813 g of N2(g) in the reaction vessel, calculate the number of moles of O2(g) left after the reaction. [5] Calculate the percentage excess O2(g) fed to the vessel. [1] If the pressure in the vessel is 250. kPa (abs) after the reaction, calculate the partial pressures of O2(g) and CO(g). [5] i. ii. iii. iv.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


