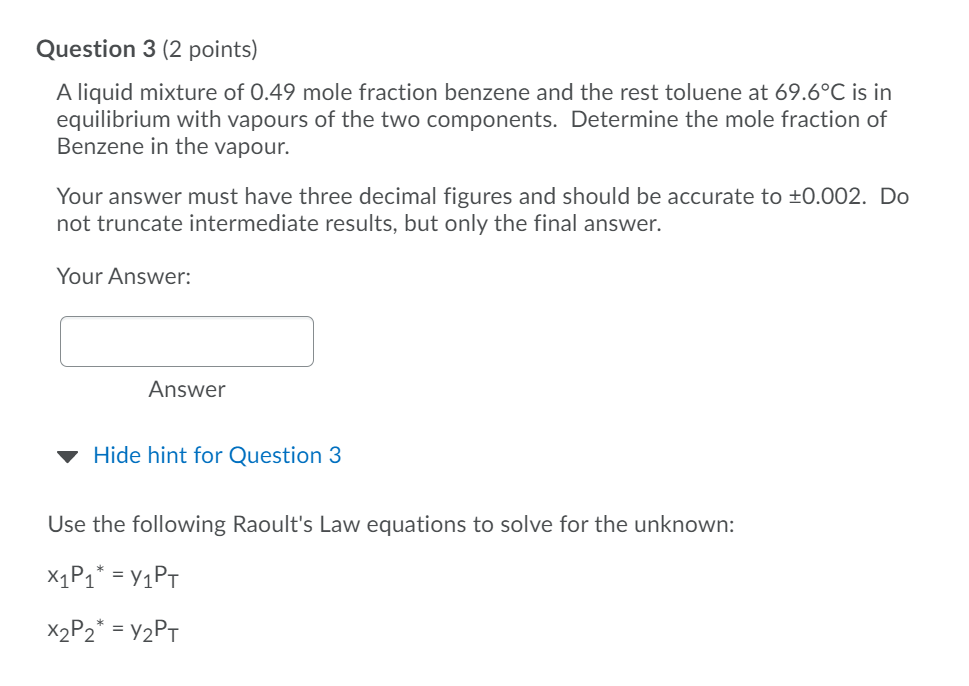
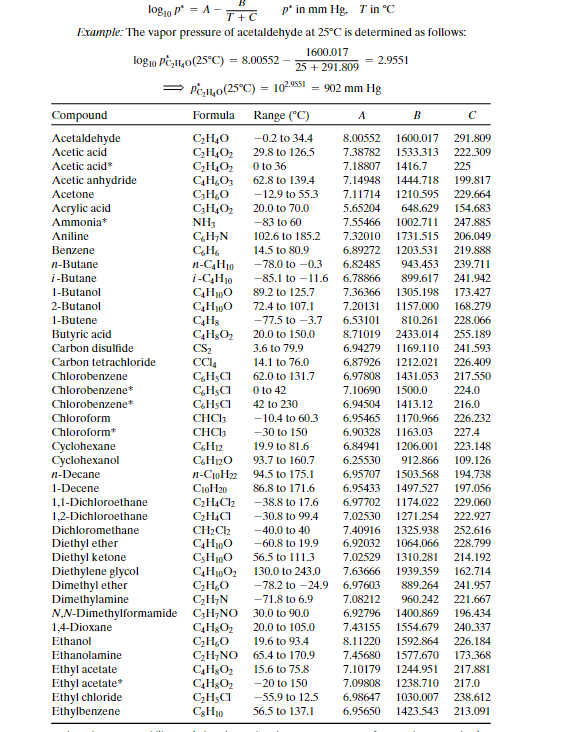
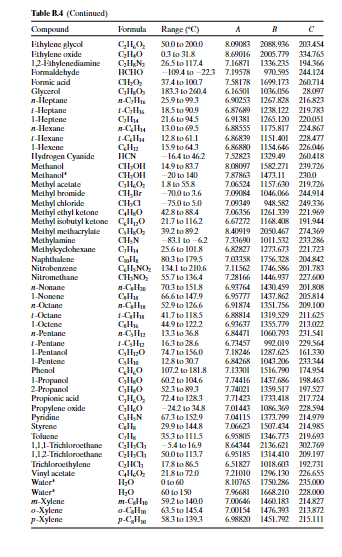
Question 3 (2 points) A liquid mixture of 0.49 mole fraction benzene and the rest toluene at 69.6C is in equilibrium with vapours of the two components. Determine the mole fraction of Benzene in the vapour. Your answer must have three decimal figures and should be accurate to +0.002. Do not truncate intermediate results, but only the final answer. Your Answer: Answer Hide hint for Question 3 Use the following Raoult's Law equations to solve for the unknown: X1P1* = y1PT X2P2* = y2PT logo = A- 1 + c p' in mm Hg. T in C Eixample: The vapor pressure of acetaldehyde at 25C is determined as follows: 1600.017 logoPC_H40(25C) = 8.00552 25 + 291.809 2.9551 > PC10(25C) = 102.9551 = 902 mm Hg Compound Formula Range (C) A B Acetaldehyde CHO -0.2 to 34.4 8.00552 1600.017 291.809 Acetic acid C2H4O2 29.8 to 1265 7.38782 1533.313 222.309 Acetic acid CH4O2 0 to 36 7.18807 1416.7 225 Acetic anhydride C4H603 62.8 to 139.4 7.14948 1444.718 199.817 Acetone CHO -12.9 to 55.3 7.11714 1210.595 229.664 Acrylic acid CH,02 20.0 to 70.0 5.65204 648.629 154.683 Ammonia NH3 -83 to 60 7.55466 1002.711 247.885 Aniline CHYN 102.6 to 185.2 7.32010 1731.515 206.049 Benzene CH 14.5 to 80.9 6.89272 1203.531 219.888 n-Butane n-CH10 -78.0 to -0.3 6.82485 943.453 239.711 i-Butane i-C4H10 -85.1 to -11.6 6.78866 899.617 241.942 1-Butanol C4H100 89.2 to 125.7 7.36366 1305.198 173.427 2-Butanol C4H100 72.4 to 107.1 7.20131 1157.000 168.279 1-Butene CH3 -77.5 to -3.7 6.53101 810.261 228.066 Butyric acid C4H302 20.0 to 150.0 8.71019 2433.014 255.189 Carbon disulfide CS 3.6 to 79.9 6.94279 1169.110 241.593 Carbon tetrachloride CCM 14.1 to 76.0 6.87926 1212.021 226.409 Chlorobenzene CHa 62.0 to 131.7 6.97808 1431.053 217.550 Chlorobenzenet CHa 0 to 42 7.10690 1500.0 224.0 Chlorobenzene C.HSC 42 to 230 6.94504 1413.12 216.0 Chloroform -10.4 to 60.3 6.95465 1170.966 226.232 Chloroform CHCl3 -30 to 150 6.90328 1163.03 227.4 Cyclohexane C6H12 19.9 to 81.6 6.84941 1206.001 223.148 Cyclohexanol CH120 93.7 to 160.7 6.25530 912.866 109.126 n-Decane n-CH22 94.5 to 175.1 6.95707 1503.568 194.738 1-Decene CroH20 86.8 to 171.6 6.95433 1497.527 197.056 1,1-Dichloroethane C2H4Cl2 -38.8 to 17.6 6.97702 1174.022 229.060 1,2-Dichloroethane C2H4a -30.8 to 99.4 7.02530 1271.254 222.927 Dichloromethane CH2Cl2 -40.0 to 40 7.40916 1325.938 252.616 Diethyl ether C4H100 -60.8 to 19.9 6.92032 1064.066 228.799 Diethyl ketone C5H100 56.5 to 1113 7.02529 1310.281 214.192 Diethylene glycol CH,130.0 to 243.0 7.63666 1939.359 162.714 Dimethyl ether CHO -78.2 to -24.9 6.97603 889.264 241.957 Dimethylamine CzH7N -71.8 to 6.9 7.08212 960.242 221.667 N,N-Dimethylformamide CzH7NO 30.0 to 90.0 6.92796 1400.869 196.434 1,4-Dioxane C4H802 20.0 to 105.0 7.43155 1554.679 240.337 Ethanol CHO 19.6 to 93.4 8.11220 1592.864 226.184 Ethanolamine CzH7NO 65.4 to 170.9 7.45680 1577.670 173.368 Ethyl acetate CH:02 15.6 to 75.8 7.10179 1244.951 217.881 Ethyl acetate* CH3O2 -20 to 150 7.09808 1238.710 217.0 Ethyl chloride CHa -55.9 to 12.5 6.98647 1030.007 238.612 Ethylbenzene CH, 56.5 to 137.1 6.95650 1423.543 213.091 Table 14 (Continued) Compound Formula Range("C) A B C Lathylene glycol GILO, SOLO to 200.0 8.09083 2088.936 203.454 Lahylene oxide C10 0.3 to 318 8.69016 2005.779 334.765 1.2.1thylenediamine CHEN 265 to 117.4 7.16871 1336.225 194.366 Formaldehyde HICHO -109.40-22.3 7.19578 970.595 244.124 Homic acid CHO 37.4 to 100,7 7.58178 1699.173 260.714 Glycerol CHO. 1833 to 261.4 6.16SOI 1036056 28.097 -leptane --CHIC 25.9 to 99.3 6.90253 1267.828 216823 1-Heplane 1-C 18.5 to 90.9 6.87689 1238.122 219.783 1-Heplene C14 21.6 94.5 6.91381 1265.120 220.051 -Hexane 13.0 to 695 6885SS 1175.817 224.867 I-Hexane I-CIR 12.8 to 611 6.86839 1151.401 228.477 1-Hexene CH 15.9 L 64.3 6.86880 1154.646 226.046 Hydrogen Cyanide HCN 16.4 to 46.2 7.52823 1329.49 260.418 Methanol CHON 14.9 to 83.7 8.08097 1582.271 279.726 Methanol -20 to 140 7.87863 1473.11 2300 Methyl acelale C11.0 1.8 to SS8 7.06524 1157.630 219.726 Methylbromide CH, Br -700 to 3.6 7.09084 1046.166 244.914 Methyl chloride CH, - 75.0 to 5.0 7.09349 948.52 249.336 Methyl ethyl ketone GIO 428 to 88.4 7.06356 1261.339 221.969 Methylisobutyl kelone CIO 21.7 to 1162 6.67272 1168.48 191.944 Mc I methacrylate CILO 39.2 to 892 8.40919 2050.467 274.369 Methylamine CHIN -83.1 to 62 7.33690 1011.532 223.286 Methylcyclohexane C14 25.6 to 101.8 6.82827 1273.673 221.721 Naphthalene CH, SL3 to 179.5 7.0075 1756.328 214.842 Nitrobenzene CHNO), 134.1 to 210.6 7.11562 1746.586 201.783 Nitromethane CHINO, 55. 7 to 136.4 728166 1446.937 227.600 -Nonane n-C 70.3 to 151.8 6.93764 1430.459 201. SOS 1-Nonene CH 66.6 to 147.9 6.95777 1437.862 205814 --Octane 52.9 L 126.6 6.91874 1351.756 219.100 I-Octane I- 41.7 to 118.5 6.88814 1319.529 211.625 1-Octene CI 44.9 to 1222 6.93637 1355.779 213.002 -Penlane -CH2 13.3 to 368 6.84471 1060.793 231.541 1-Penlane I-CHEZ 16.3 to 286 6.73457 992.019 229.564 1-Pentanal CHO 74.7 to 1560 7.18246 1287.625 161.330 1-Pentene CH 12.8 to 30.7 6.84268 1043.216233344 Phenol CH10 107.2 to 18. 7.13301 1516.790 174.954 1-Propanol CHO GOL2 to 104.6 7.74416 1437.686 198.463 2-Propanol CILO 52.389.3 7.74021 1359.517 197.527 Propionic acid CHO, 72.4 to 128.3 7.71423 1723.418 217.724 Propylene arxide CO -24.2 to 34.8 7.01443 1086.369 228.594 Pyridine CHEN 673 to 1529 7,04115 1373.799 214.979 Styrene CH 29.9 to 144.8 7.06623 1507.434 214.985 Toluene CIL 35.3 to 111.5 6.95805 1346.773 219.693 1.1.1-'Trichowethane CICH -5.4 10 16.9 8.64344 2136.621 302.769 1,12-Trichlorethane CH.CH SOLO to 1137 6.9518S 1314.410 209.197 Trichloroethylene CHCL 17.810 865 6.51827 1018.63 192.731 Vinyl acetate GH02 21.8 L 720 721010 1296.130 226655 Wailer HO Oto 60 8.10765 1750.286 235.000 Water HO 60 to 150 7.96681 1668.210 228.000 m-Xylene m-CHI 59.2 to 140.0 7.00646 1460.183 214.827 o-Xylene 0-CU 435 144 7.00154 1476.393 213.872 p-Xylene p-CHE 58.3 to 119.3 6.98820 1451.792 215.111









