Answered step by step
Verified Expert Solution
Question
1 Approved Answer
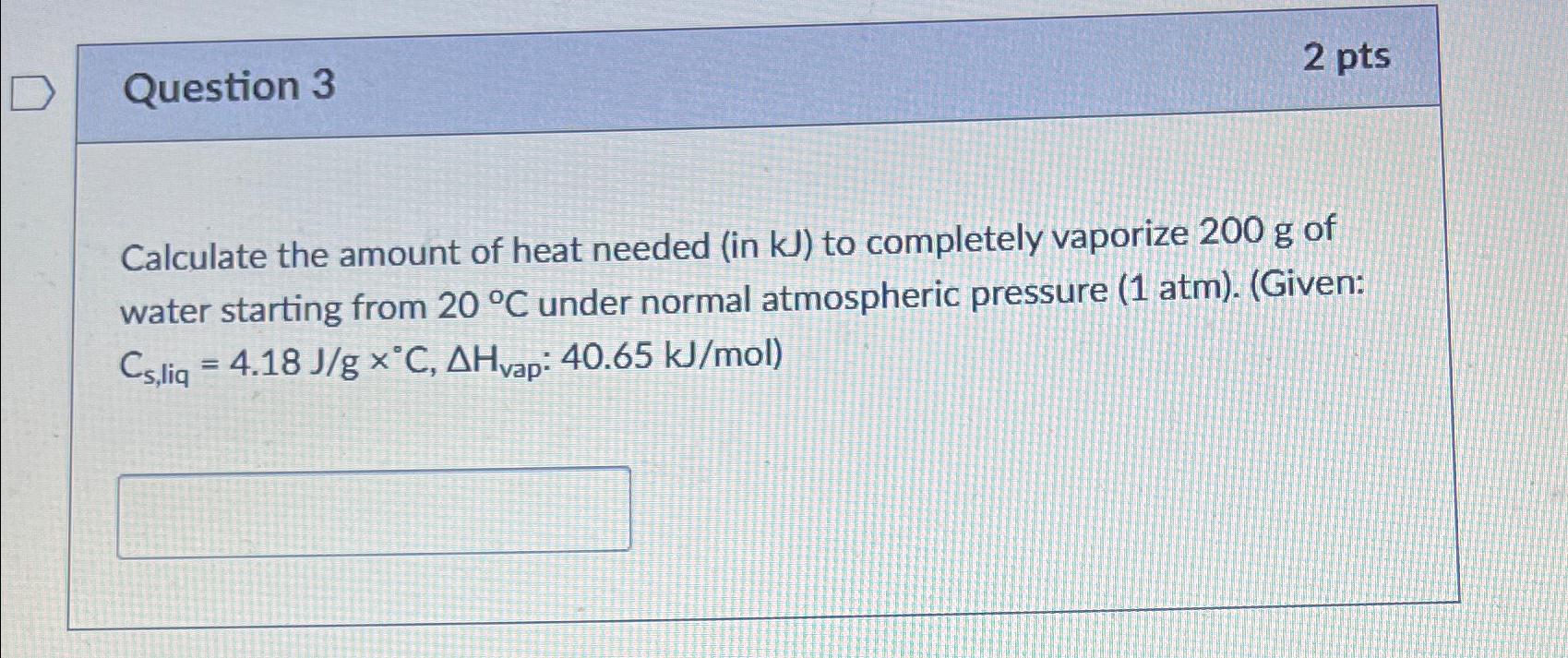
Question 3 2 pts Calculate the amount of heat needed (in kJ ) to completely vaporize 200g of water starting from 20deg C under normal
Question 3\ 2 pts\ Calculate the amount of heat needed (in
kJ) to completely vaporize
200gof water starting from
20\\\\deg Cunder normal atmospheric pressure
(1atm). (Given:
C_(s,liq )=4.18(J)/(g)\\\\times \\\\deg C,\\\\Delta H_(vap ):40.65k(J)/(m)ol)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


