Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 3: a) Dehydrogenation is an important and a useful reaction in such a way that it converts alkanes into low valued olefins which are
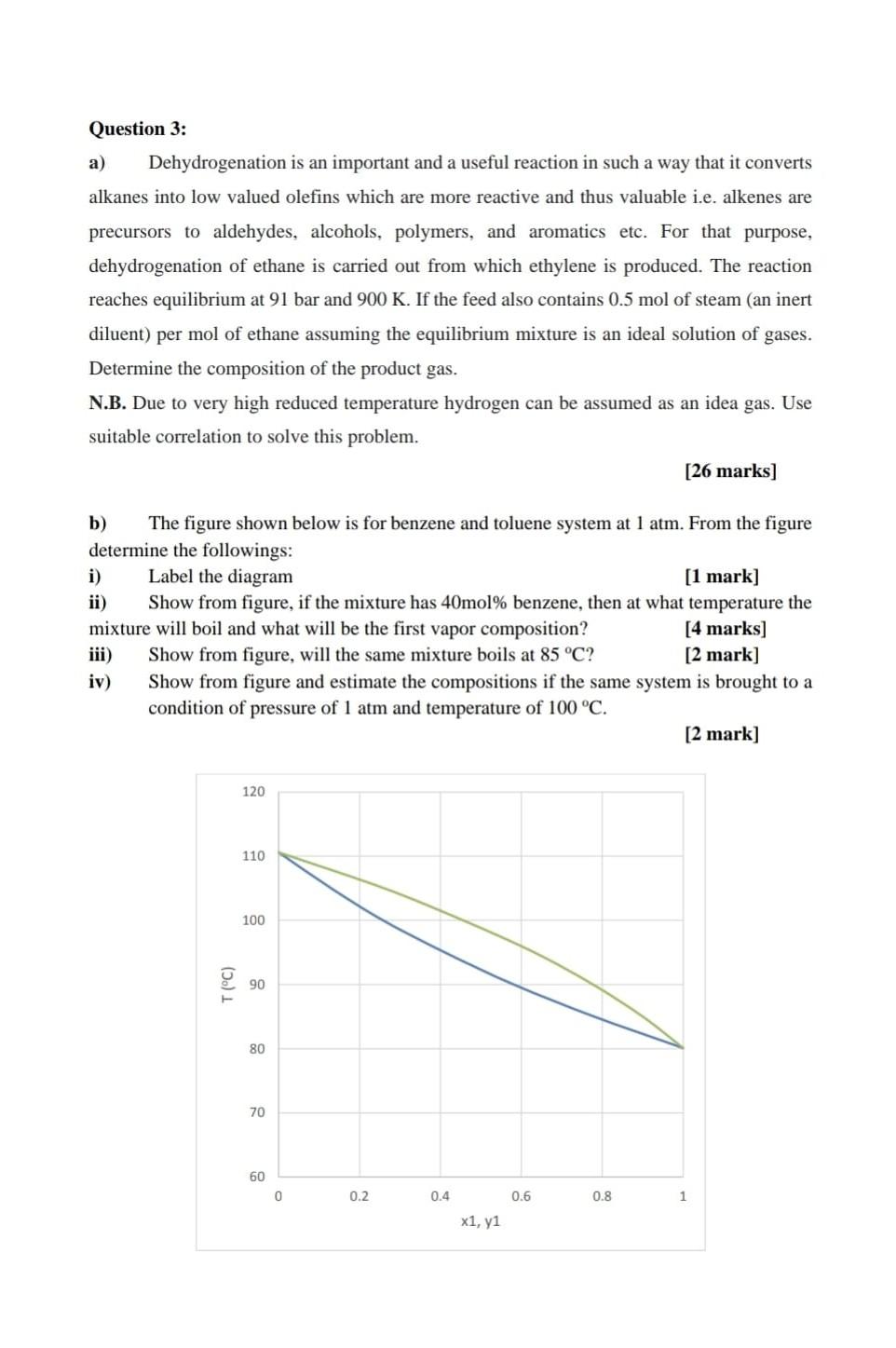
Question 3: a) Dehydrogenation is an important and a useful reaction in such a way that it converts alkanes into low valued olefins which are more reactive and thus valuable i.e. alkenes are precursors to aldehydes, alcohols, polymers, and aromatics etc. For that purpose, dehydrogenation of ethane is carried out from which ethylene is produced. The reaction reaches equilibrium at 91 bar and 900 K. If the feed also contains 0.5 mol of steam (an inert diluent) per mol of ethane assuming the equilibrium mixture is an ideal solution of gases. Determine the composition of the product gas. N.B. Due to very high reduced temperature hydrogen can be assumed as an idea gas. Use suitable correlation to solve this problem. [26 marks] b) The figure shown below is for benzene and toluene system at 1 atm. From the figure determine the followings: i) Label the diagram [1 mark] ii) Show from figure, if the mixture has 40mol% benzene, then at what temperature the mixture will boil and what will be the first vapor composition? [4 marks] Show from figure, will the same mixture boils at 85 C? [2 mark] iv) Show from figure and estimate the compositions if the same system is brought to a condition of pressure of 1 atm and temperature of 100 C. [2 mark] 120 110 100 90 80 70 60 0 0.2 0.4 0.6 0.8 1 x1, y1
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


