Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider The Following Spectrum Of Haloduracin (elemental Formula: C297 H479 N85 099 S5). peak m/z Rel. Intensity 1 2327.796734 9.72247531 2 2328.130883 35.55559953 3 2328.465183
Consider The Following Spectrum Of Haloduracin (elemental Formula: C297 H479 N85 099 S5).
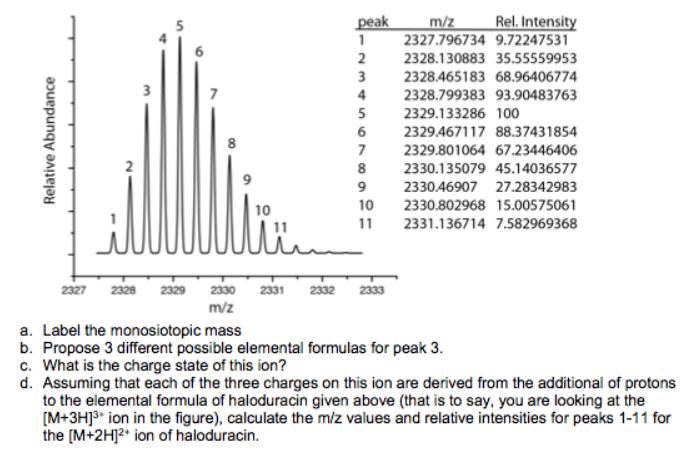
peak m/z Rel. Intensity 1 2327.796734 9.72247531 2 2328.130883 35.55559953 3 2328.465183 68.96406774 7 4 2328.799383 93.90483763 5 2329.133286 100 2329.467117 88.37431854 7 2329.801064 67.23446406 2330.135079 45.14036577 9 2330.46907 27.28342983 10 2330.802968 15.00575061 10 11 2331.136714 7.582969368 2327 2328 2329 2330 2331 2332 2333 m/z a. Label the monosiotopic mass b. Propose 3 different possible elemental formulas for peak 3. c. What is the charge state of this ion? d. Assuming that each of the three charges on this ion are derived from the additional of protons to the elemental formula of haloduracin given above (that is to say, you are looking at the [M+3HJ3* ion in the figure), calculate the miz values and relative intensities for peaks 1-11 for the [M+2H]2 ion of haloduracin. Relative Abundance
Step by Step Solution
★★★★★
3.28 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
Solution Ans a Monoisotopic mass is 2327796734 with 927 intensity Ans b ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


