Question
Pure caffeine has a melting point of around 235 C. Suppose after you isolated your caffeine from coffee, you found that your sample had
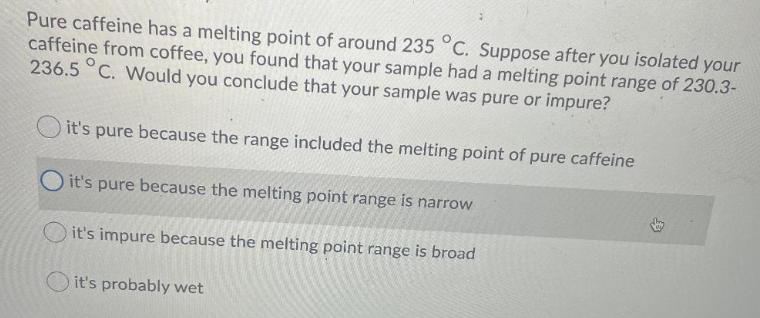
Pure caffeine has a melting point of around 235 C. Suppose after you isolated your caffeine from coffee, you found that your sample had a melting point range of 230.3- 236.5 C. Would you conclude that your sample was pure or impure? it's pure because the range included the melting point of pure caffeine Oit's pure because the melting point range is narrow it's impure because the melting point range is broad it's probably wet
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Question 391 has a sharp melt A Compound material shapp melting point if the mange of m...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Graham Solomons, Craig Fryhle, Scott Snyder
11th edition
1118133579, 978-1118133576
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



