Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 4 please Chem 373 Spectroscopy Assignment Fall 2002 An organic chemist was asked to identify an amber liquid with a sweet odot. To accomplish
Question 4 please 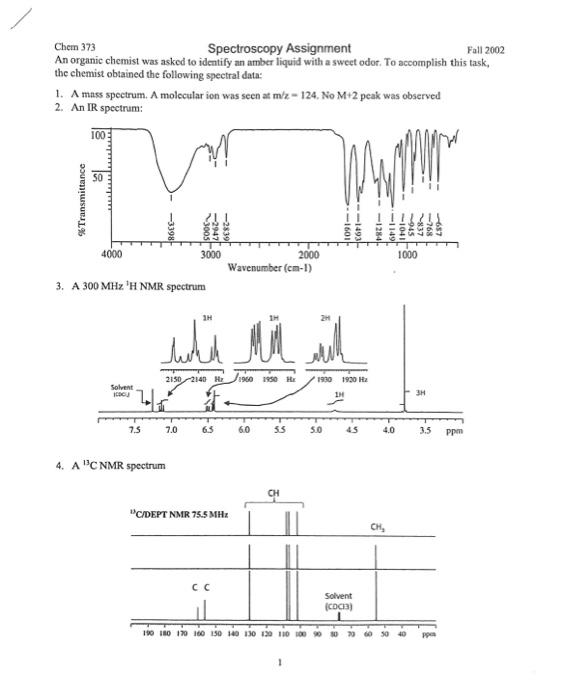
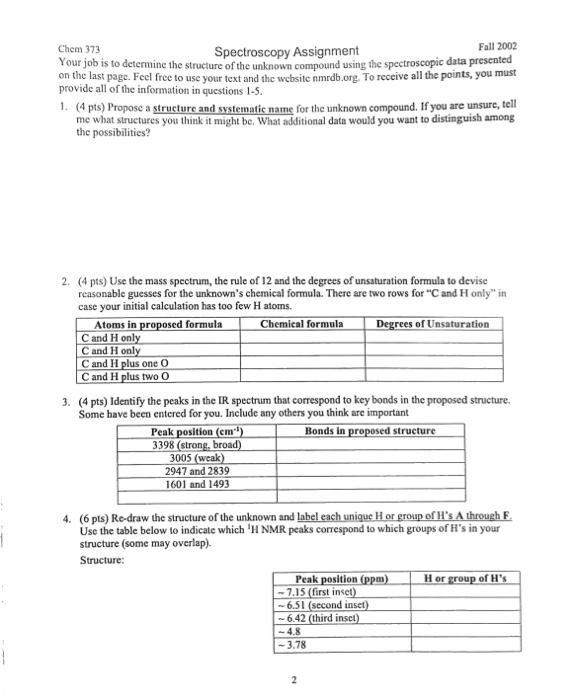
Chem 373 Spectroscopy Assignment Fall 2002 An organic chemist was asked to identify an amber liquid with a sweet odot. To accomplish this task, the chemist obtained the following spectral data: 1. A mass speetrum. A molecular ion was seen at m/z=124, No M+2 peak was observed 2. An IR spectrum: 3. A 300MHz1H NMR spectrum 4. A13C NMR spectrum Chen 373 Spectroscopy Assignment Fall 2002 Your job is to determine the structure of the unknown compound using the spectroscopic data presented on the last page. Feel free to use your text and the website amrdb.org. To receive all the points, you must provide all of the information in questions 1-5. 1. (4 pts) Propose a structure and systematic name for the unknown compound. If you are unsure, tell me what struetures you think it might be. What additional datn would you want to distinguish among the possibilities? 2. (4 pts) Use the mass spectrum, the rule of 12 and the degrees of unsaturation formula to devise reasonable guesses for the unknown's chemical formula. There are two rows for " C and H only" in case your initial calculation has too few H atoms. 3. (4 pts) Identify the peaks in the IR spectrum that correspond to key bonds in the proposed structure. Some have been entered for you. Inelude any others you think are important 4. ( 6 pts) Re-draw the structure of the unknown and label each unigue H or group of H 's A through F. Use the table below to indicate which 'H NMR peaks correspond to which groups of H's in your structure (some may overlap). Structure: Chem 373 Spectroscopy Assignment Fall 2002 An organic chemist was asked to identify an amber liquid with a sweet odot. To accomplish this task, the chemist obtained the following spectral data: 1. A mass speetrum. A molecular ion was seen at m/z=124, No M+2 peak was observed 2. An IR spectrum: 3. A 300MHz1H NMR spectrum 4. A13C NMR spectrum Chen 373 Spectroscopy Assignment Fall 2002 Your job is to determine the structure of the unknown compound using the spectroscopic data presented on the last page. Feel free to use your text and the website amrdb.org. To receive all the points, you must provide all of the information in questions 1-5. 1. (4 pts) Propose a structure and systematic name for the unknown compound. If you are unsure, tell me what struetures you think it might be. What additional datn would you want to distinguish among the possibilities? 2. (4 pts) Use the mass spectrum, the rule of 12 and the degrees of unsaturation formula to devise reasonable guesses for the unknown's chemical formula. There are two rows for " C and H only" in case your initial calculation has too few H atoms. 3. (4 pts) Identify the peaks in the IR spectrum that correspond to key bonds in the proposed structure. Some have been entered for you. Inelude any others you think are important 4. ( 6 pts) Re-draw the structure of the unknown and label each unigue H or group of H 's A through F. Use the table below to indicate which 'H NMR peaks correspond to which groups of H's in your structure (some may overlap). Structure 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


