Answered step by step
Verified Expert Solution
Question
1 Approved Answer
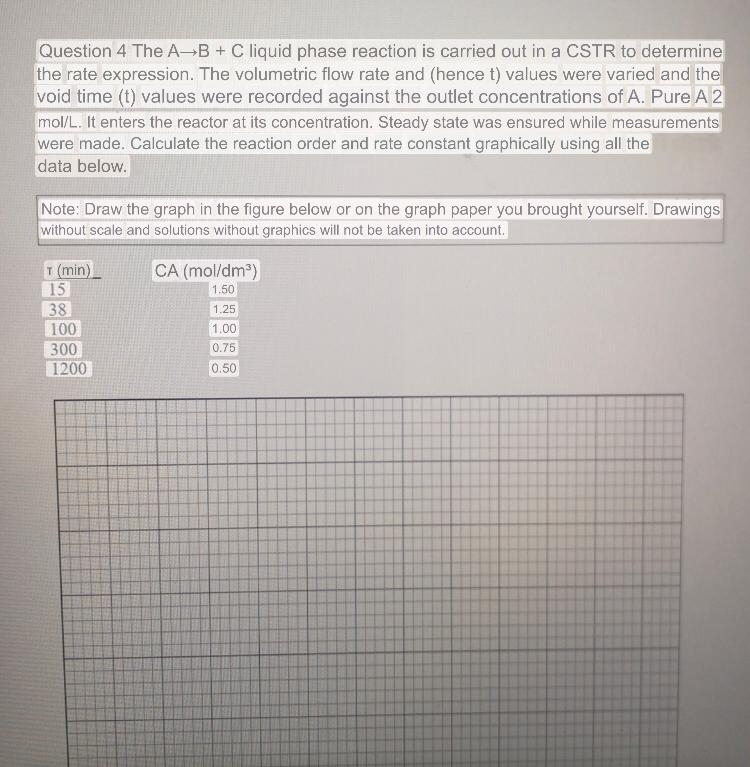
Question 4 The A B + C liquid phase reaction is carried out in a CSTR to determine the rate expression. The volumetric flow rate
Question The liquid phase reaction is carried out in a CSTR to determine the rate expression. The volumetric flow rate and hence values were varied and the void time values were recorded against the outlet concentrations of A Pure A It enters the reactor at its concentration. Steady state was ensured while measurements were made. Calculate the reaction order and rate constant graphically using all the data below.
Note: Draw the graph in the figure below or on the graph paper you brought yourself. Drawings without scale and solutions without graphics will not be taken into account.
table
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


