Question 4, the given pH being 7.10. Determine the answer using the Henderson-Hasselbach equation for the [HA]/[A-] ratio.
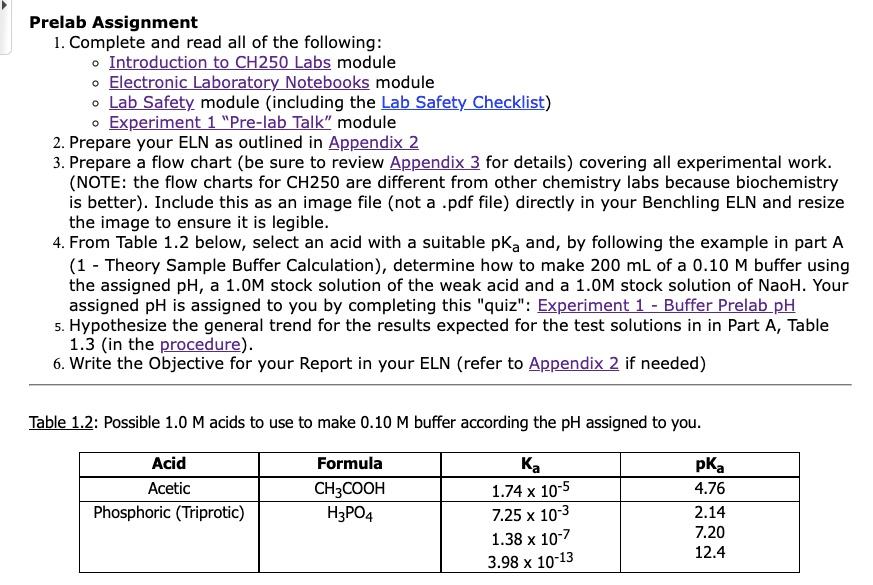
Prelab Assignment 1. Complete and read all of the following: - Introduction to CH250 Labs module Electronic Laboratory Notebooks module Lab Safety module (including the Lab Safety Checklist) Experiment 1 "Pre-lab Talk" module 2. Prepare your ELN as outlined in Appendix 2 3. Prepare a flow chart (be sure to review Appendix 3 for details) covering all experimental work. (NOTE: the flow charts for CH250 are different from other chemistry labs because biochemistry is better). Include this as an image file (not a .pdf file) directly in your Benchling ELN and resize the image to ensure it is legible. 4. From Table 1.2 below, select an acid with a suitable pk, and, by following the example in part A (1 - Theory Sample Buffer Calculation), determine how to make 200 mL of a 0.10 M buffer using the assigned pH, a 1.0M stock solution of the weak acid and a 1.0M stock solution of Naoh. Your assigned pH is assigned to you by completing this "quiz": Experiment 1 - Buffer Prelab pH 5. Hypothesize the general trend for the results expected for the test solutions in in Part A, Table 1.3 (in the procedure). 6. Write the Objective for your Report in your ELN (refer to Appendix 2 if needed) Table 1.2: Possible 1.0 M acids to use to make 0.10 M buffer according the pH assigned to you. Acid Acetic Phosphoric (Triprotic) Formula CH3COOH H3PO4 1.74 x 10-5 7.25 x 10-3 1.38 x 10-7 x 3.98 x 10-13 4.76 2.14 7.20 12.4 Prelab Assignment 1. Complete and read all of the following: - Introduction to CH250 Labs module Electronic Laboratory Notebooks module Lab Safety module (including the Lab Safety Checklist) Experiment 1 "Pre-lab Talk" module 2. Prepare your ELN as outlined in Appendix 2 3. Prepare a flow chart (be sure to review Appendix 3 for details) covering all experimental work. (NOTE: the flow charts for CH250 are different from other chemistry labs because biochemistry is better). Include this as an image file (not a .pdf file) directly in your Benchling ELN and resize the image to ensure it is legible. 4. From Table 1.2 below, select an acid with a suitable pk, and, by following the example in part A (1 - Theory Sample Buffer Calculation), determine how to make 200 mL of a 0.10 M buffer using the assigned pH, a 1.0M stock solution of the weak acid and a 1.0M stock solution of Naoh. Your assigned pH is assigned to you by completing this "quiz": Experiment 1 - Buffer Prelab pH 5. Hypothesize the general trend for the results expected for the test solutions in in Part A, Table 1.3 (in the procedure). 6. Write the Objective for your Report in your ELN (refer to Appendix 2 if needed) Table 1.2: Possible 1.0 M acids to use to make 0.10 M buffer according the pH assigned to you. Acid Acetic Phosphoric (Triprotic) Formula CH3COOH H3PO4 1.74 x 10-5 7.25 x 10-3 1.38 x 10-7 x 3.98 x 10-13 4.76 2.14 7.20 12.4







