Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 6 1 pts 514) of thermal energy is added to a 155 g sample of lead that is initially at 25.0C. What will be
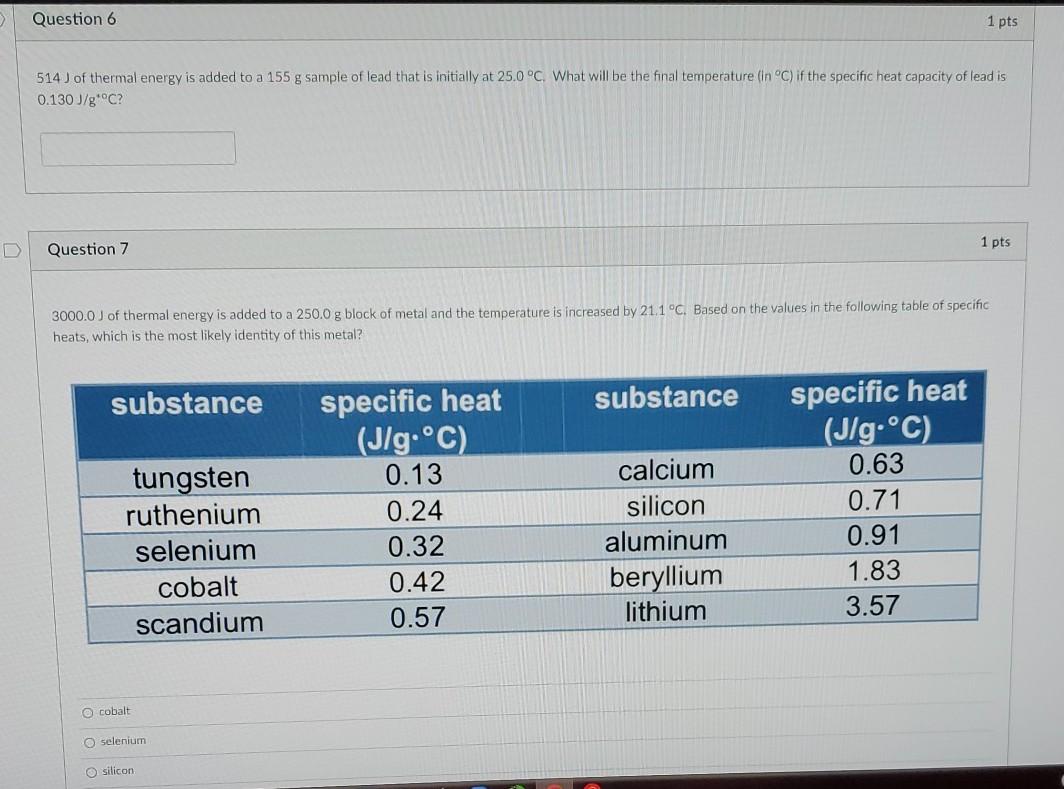
Question 6 1 pts 514) of thermal energy is added to a 155 g sample of lead that is initially at 25.0C. What will be the final temperature (in C) if the specific heat capacity of lead is 0.130 J/g C? Question 7 1 pts 3000.0 J of thermal energy is added to a 250,0 g block of metal and the temperature is increased by 21.1 C. Based on the values in the following table of specific heats, which is the most likely identity of this metal? substance substance tungsten ruthenium selenium cobalt scandium specific heat (J/g.C). 0.13 0.24 0.32 0.42 0.57 calcium silicon aluminum beryllium lithium specific heat (J/g.C) 0.63 0.71 0.91 1.83 3.57 cobalt selenium silicon
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


